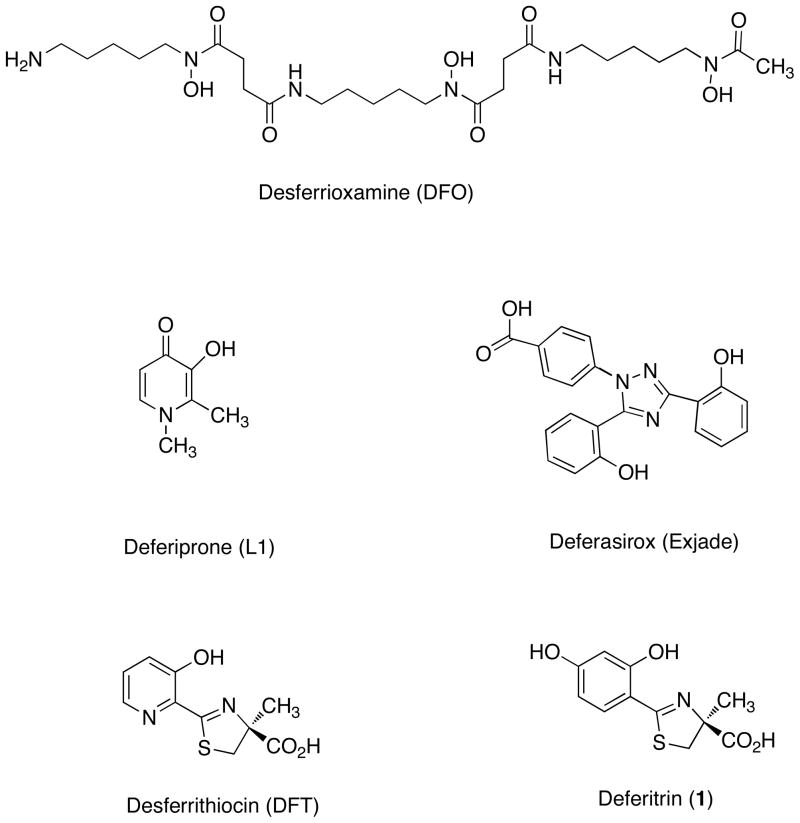
Figure 1.
Structures of iron chelators that are now in use or that have been clinically evaluated: desferrioxamine B mesylate (DFO), 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone, L1), 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoic acid (deferasirox, Exjade), and the desferrithiocin [(S)-4,5-dihydro-2-(3-hydroxy-2-pyridinyl)-4-methyl-4-thiazolecarboxylic acid (DFT)] analogue (S)-4,5-dihydro-2-(2,4-dihydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid, deferitrin (1).