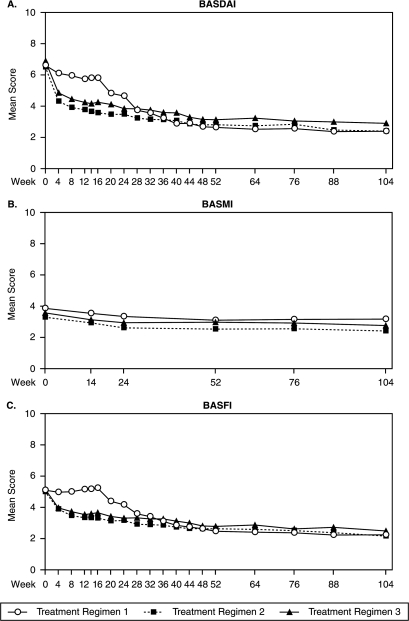
Figure 4.
Mean BASDAI, BASMI and BASFI scores through week 104. Treatment regimen 1 consisted of patients who were originally assigned to placebo at baseline and who either entered early escape at week 16 to receive golimumab 50 mg through week 104 or crossed over at week 24 to receive golimumab 50 mg through week 104. Treatment regimen 2 consisted of patients who were originally assigned to golimumab 50 mg at baseline and who either entered early escape at week 16 to receive golimumab 100 mg through week 104 or continued 50 mg through week 104. Treatment regimen 3 consisted of patients who were originally assigned to golimumab 100 mg at baseline and who did not receive study medication adjustments. Observed data are presented without imputation. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index.