Abstract
We recently established an immunohistochemical method for the detection of blue light (BL)-induced and phototropin-mediated phosphorylation of plasma-membrane H+-ATPase in stomatal guard cells of Arabidopsis thaliana. This technique makes it possible to detect the phosphorylation/activation status of guard-cell H+-ATPase in the epidermis of a single rosette leaf, without the need to prepare guard-cell protoplasts (GCPs) from a large number of plants. Moreover, it can detect guard-cell responses under more natural and stress-free conditions compared to using GCPs. Taking advantage of these properties, we examined the effect of abscisic acid (ABA) on BL-induced phosphorylation of guard-cell H+-ATPase by using ABA-insensitive mutants. This revealed inhibition of BL-induced phosphorylation of guard-cell H+-ATPase via the early ABA-signaling components PYR/PYL/RCAR-PP2Cs-SnRK2s, which are known to be early ABA-signaling components for a wide range of ABA responses in plants.
Keywords: ABA, Blue light, Guard cell, Immunohistochemistry, phosphorylation, Plasma membrane H+-ATPase
Immunohistochemical detection of blue-light-induced phosphorylation of plasma-membrane H+-ATPase in guard cells
Stomatal pores surrounded by a pair of guard cells in the epidermis regulate gas exchange between leaves and the atmosphere. The opening of stomata is induced by blue light (BL) and closing is induced by the phytohormone abscisic acid (ABA), which is synthesized in response to drought stress. ABA signaling is thought to predominate over BL signaling in guard cells, since it is important for plants to prevent water loss under drought stress, even in sunlight.1,2 The molecular mechanism by which ABA inhibits BL signaling in guard cells, however, has yet to be elucidated.
The BL receptor phototropin (phot1 and phot2) mediates activation of plasma-membrane H+-ATPase in response to BL through phosphorylation of a penultimate threonine (Thr) in the C-terminus, concomitant with binding of the 14–3-3 protein to the phosphorylated C-terminus in stomatal guard cells.3,4 Recent investigation indicates that FLOWERING LOCUS T acts as a positive regulator for stomatal opening via activation of H+-ATPase.5 Activated H+-ATPase creates an inside-negative electrical potential across the plasma membrane that allows the influx of K+ into guard cells and leads to stomatal opening.6 To date, detection of the degree of phosphorylation of guard-cell H+-ATPase has been performed biochemically by using guard-cell protoplasts (GCPs).2,7 Preparation of GCPs from Arabidopsis plants for this purpose, however, requires over 5,000 rosette leaves and takes more than 8 h. Isolation of GCPs from dwarf plants, in which the number and area of rosette leaves are small, or from several plants/mutants simultaneously is therefore difficult. In addition, GCP preparation involves harsh treatments including shredding the leaves in a blender, incubation on ice, cell-wall digestion by enzymes, centrifugation, and osmotic stress, all of which may damage or stress the guard cells.
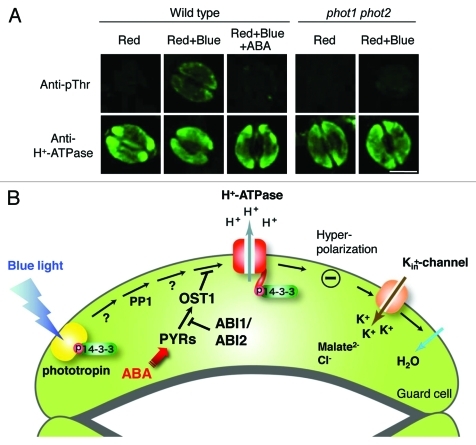
We therefore established an immunohistochemical method for the detection of BL-induced and phototropin-mediated phosphorylation of plasma membrane H+-ATPase in the epidermis of a single Arabidopsis rosette leaf using a specific antibody against the phosphorylated penultimate Thr of H+-ATPase (anti-pThr).8,9 As we expected, BL-induced phosphorylation of the H+-ATPase was detected immunohistochemically in the wild type but not in a phot1 phot2 double mutant (Fig. 1A). BL responses of guard cells in the epidermis detected by this immunohistochemical method showed almost identical properties to those of GCPs in previous studies.9
Figure 1.
(A) Immunohistochemical detection of BL-induced phosphorylation of plasma-membrane H+-ATPase in stomatal guard cells of Arabidopsis thaliana. BL-induced phosphorylation of guard-cell H+-ATPase was not observed in epidermis from the phototropin double mutant (phot1 phot2). Also, 20 µM ABA completely inhibited phosphorylation of H+-ATPase. The epidermis was isolated from Arabidopsis rosette leaves of the wild type and phot1 phot2 and incubated under red light (50 µmol m−2 s−1) for 20 min (Red), then BL (10 µmol m−2 s−1) was superimposed on background red light for 2.5 min (Red+Blue). ABA (20 µM) was simultaneously added to the epidermis illuminated with red light for 20 min (Red+Blue+ABA). For irradiation of the epidermal fragments, both red light and BL were obtained using light-emitting photodiodes. Upper panels show typical fluorescence images of the phosphorylation of H+-ATPase by anti-pThr and lower panels show corresponding typical fluorescence images by anti-H+-ATPase in the epidermis. Scale bar = 10 µm. (B) Schematic representation of crosstalk of the BL- and ABA-signaling pathways in stomatal guard cells. BL-induced phosphorylation of H+-ATPase was completely inhibited by a physiological concentration of ABA via the early ABA-signaling pathway including PYR/PYL/RCAR-PP2Cs-SnRK2s. Note that it has been suggested that phosphatidic acid, a downstream ABA-signaling component, inhibits BL-induced phosphorylation of H+-ATPase via inhibition of type 1 protein phosphatase (PP1), which is a positive signaling component between phototropin and H+-ATPase.19-21 Abbreviations: ABA, abscisic acid; ABI1, ABA-insensitive 1; ABI2, ABA-insensitive 2; OST1, OPEN STOMATA 1; PP1, type 1 protein phosphatase; PYRs, ABA receptor pyrabactin resistant.
Physiological concentrations of ABA completely inhibit blue-light-induced phosphorylation of guard-cell H+-ATPase
In our experiments, a physiological concentration of ABA (1 µM) completely inhibited BL-induced phosphorylation of guard-cell H+-ATPase in the epidermis. We also noted that the epidermis was more sensitive to inhibition by ABA than was shown previously for GCPs.9 These results demonstrate that this immunohistochemical method is very useful for detecting the phosphorylation status of guard-cell H+-ATPase under more natural and stress-free conditions compared with using GCPs.
Elucidation of crosstalk between the BL- and ABA-signaling pathways in guard cells is very important for understanding physiological guard cell-responses under drought stress, even in sunlight. To clarify the molecular mechanism of crosstalk between BL and ABA, we applied this technique to the ABA-insensitive mutants abi1–1, abi2–1, and ost1–2.10-15 Our results show that ABA at 20 µM had no effect on BL-induced phosphorylation in these mutants. It has been demonstrated that ABA-insensitive 1 and 2 (ABI1 and ABI2) are type 2C protein phosphatases (PP2Cs) and OPEN STOMATA 1 (OST1) is a protein kinase within the SnRK2 protein family. All three act as early ABA-signaling components that interact with the ABA-receptors pyrabactin resistance/pyrabactin resistance 1-like/regulatory component of ABA receptor (PYR/PYL/RCAR).16-18 Taken together, this suggests that ABA inhibits BL-induced activation of H+-ATPase via an early ABA-signaling pathway that includes PYR/PYL/RCAR-PP2Cs-SnRK2s (Fig. 1B). To our knowledge, this is the first evidence of the involvement of PYR/PYL/RCAR-PP2Cs-SnRK2s in the inhibition of BL-induced phosphorylation of guard-cell H+-ATPase by ABA.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17800
References
- 1.Shimazaki K, Doi M, Assmann SM, Kinoshita T. Light regulation of stomatal movement. Annu Rev Plant Biol. 2007;58:219–47. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–91. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–60. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita T, Shimazaki K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999;18:5548–58. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, et al. FLOWERING LOCUS T regulates stomatal opening. Curr Biol. 2011;21:1232–8. doi: 10.1016/j.cub.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita T, Hayashi Y. New insights into the regulation of stomatal opening by blue light and the plasma membrane H+-ATPase. Int Rev Cell Mol Biol. 2011;289:89–115. doi: 10.1016/B978-0-12-386039-2.00003-1. [DOI] [PubMed] [Google Scholar]
- 7.Ueno K, Kinoshita T, Inoue S, Emi T, Shimazaki K. Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 2005;46:955–63. doi: 10.1093/pcp/pci104. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T. Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol. 2010;51:1186–96. doi: 10.1093/pcp/pcq078. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Inoue S, Takahashi K, Kinoshita T. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 2011;52:1238–48. doi: 10.1093/pcp/pcr072. [DOI] [PubMed] [Google Scholar]
- 10.Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–52. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- 11.Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–71. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–5. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 13.Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001;25:295–303. doi: 10.1046/j.1365-313x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 14.Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002;30:601–9. doi: 10.1046/j.1365-313X.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 15.Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–99. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–8. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010;61:290–9. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita T, Shimazaki K. Involvement of calyculin A- and okadaic acid- sensitive protein phosphatase in the blue light response of stomatal guard cells. Plant Cell Physiol. 1997;38:1281–5. [Google Scholar]
- 20.Takemiya A, Kinoshita T, Asanuma M, Shimazaki K. Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba. Proc Natl Acad Sci USA. 2006;103:13549–54. doi: 10.1073/pnas.0602503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takemiya A, Shimazaki K. Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase 1. Plant Physiol. 2010;153:1555–62. doi: 10.1104/pp.110.155689. [DOI] [PMC free article] [PubMed] [Google Scholar]