Abstract
Plant volatiles include terpenoids, which are generally involved in plant defense, repelling pests and pathogens and attracting insects for herbivore control, pollination and seed dispersal. Orange fruits accumulate the monoterpene limonene at high levels in the oil glands of their fruit peels. When limonene production was downregulated in orange fruits by the transgenic expression of a limonene synthase (CitMTSE1) in the antisense configuration, these fruits were resistant to the fungus Penicillium digitatum (Pers.) Sacc. and the bacterium Xanthomonas citri subsp. citri and were less attractive to the medfly pest Ceratitis capitata. These responses were reversed when the antisense transgenic orange fruits were treated with limonene. To gain more insight into the role of the limonene concentration in fruit responses to pests and pathogens, we attempted to overexpress CitMTSE1 in the sense configuration in transgenic orange fruits. Only slight increases in the amount of limonene were found in sense transgenic fruits, maybe due to the detrimental effect that excessive limonene accumulation would have on plant development. Collectively, these results suggest that when limonene reaches peak levels as the fruit develops, it becomes a signal for pest and pathogen attraction, which facilitate access to the fruit for pulp consumers and seed dispersers.
Keywords: co-evolution, defense, repellency, secondary metabolism, seed dispersers, trophic interaction, volatiles
In recent years, large efforts have been made to understand the biosynthetic pathways regulating the production of terpene volatiles in plants as well as the metabolism and physiological effects of these compounds.1 Evaluating volatile emission as a language for communication between plants and the environment is gaining increasing interest.
Upon maturation, fleshy fruits modify their color, taste, texture and aroma to become more attractive to frugivore animals for seed dispersal. The volatile content and emission profiles undergo drastic changes during fruit development and maturation, but little is known about the roles of specific volatiles in interactions with herbivores and other seed-dispersing agents. Regarding the impact of specialized insect pests and pathogens on fleshy fruits, most studies have focused largely on pests and pathogens as competitors of seed dispersers.2-4 A broader exploration of the pathogen-plant-vertebrate relationship from the perspective of evolutionary ecology is enticing.
The monoterpene limonene represents up to 97% of the total volatiles in orange fruit peel. Previously, we have shown that overexpression of an antisense (AS) construct of a limonene synthase gene from mandarin (CitMTSE1) in transgenic oranges resulted in a downregulation of their synthesis and reduced accumulation of limonene as well as other related monoterpenes, and increased amounts of monoterpene alcohols such as nerol, geraniol and citronellol.5 AS fruits showed a marked resistance against the fungus Penicillium digitatum (Pers.) Sacc. and the bacterium Xanthomonas citri subsp citri, two important citrus fruit pathogens, which were unable to infect transgenic peel tissues. In addition, males of one of the most polyphagous pests of citrus fruits, Ceratitis capitata, were much less attracted to the AS fruits than empty vector (EV) control fruits.5 Interestingly, the resistant/less attractant phenotype was fully reversed when AS fruits were treated with exogenous limonene (and not with nerol or citronellol), indicating that limonene accumulation in the orange peel modulates fruit interactions with insects and microorganisms.
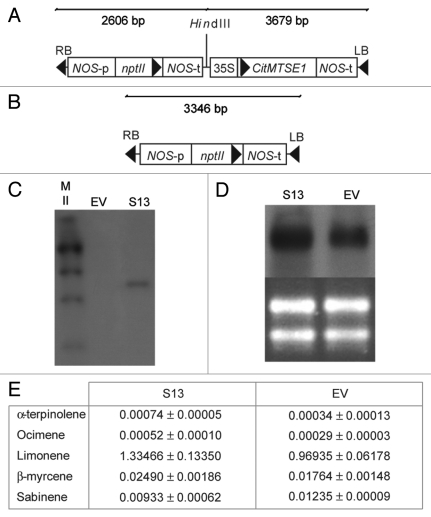
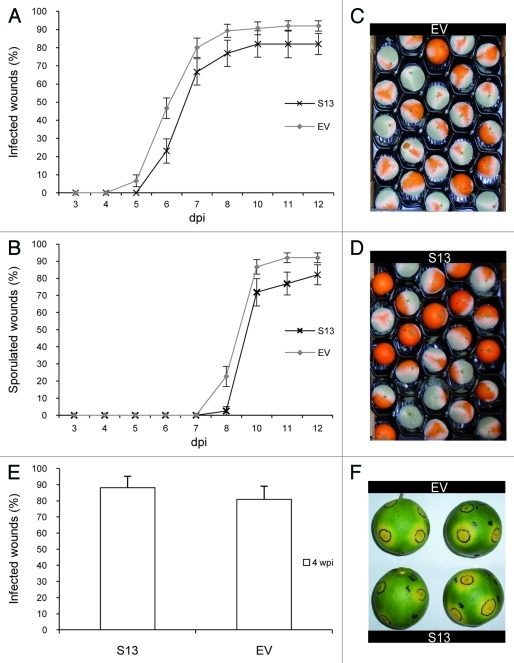
To determine whether the constitutive increase in the production of limonene could lead to a higher level of attraction of C. capitata and greater susceptibility to the tested pathogens, transgenic orange plants overexpressing the same limonene synthase gene from mandarin (CitMTSE1)6 in the sense orientation were generated (Fig. 1A–D). Limonene synthase transcript accumulation was higher in sense (S) than in EV lines (Fig. 1D). However, total terpenes profiling in fruit peels from the sense lines showed very slight increases in limonene accumulation compared with fruits from the EV control line (Fig. 1E). Although we found increases of more than 4-fold in the levels of some monoterpenes, such as α-terpinolene and δ-3-carene, in leaf tissues in S lines compared with EV controls, none of the S lines showed an increase in limonene in fruit peels of more than 1.4 times, and the levels of related mono- and sesquiterpenoid compounds remained basically unaltered (Table S1). Further, fruits of the S lines were challenged with P. digitatum and X. citri subsp citri. When mature S and EV orange fruits cv Pineapple were inoculated with P. digitatum and the percentage of infected wounds and wounds with spores in EV fruits 10 d postinoculation were 90.66% and 86.66%, respectively, as compared with 82.05% and 71.79%, respectively, in S fruits (Fig. 2A–D). S fruits also showed a response similar to that of EV fruits when challenged with X. citri subsp citri; the percentage of infected wounds at 4 weeks postinoculation in green fruits inoculated with the bacterium was 80.83% in EV fruits and 88.09% in S fruits (Fig. 2E, F) and no significant differences were found, with p < 0.05. Similar results were obtained for the S and EV lines from another transformation experiment with the Navelina orange cultivar (data not shown). Attempts to overexpress terpenoids in transgenic plants have resulted in enhanced accumulation of the expected compound in several cases,7-9 but the growth and/or development of these plants were usually affected, with the strength of the phenotype being correlated with the accumulation level of the transgenically produced terpenoid.10-12 S orange lines did not show noticeable changes in plant and fruit morphology compared with controls. It may be possible that S lines did not overproduce limonene because such high levels of production could be detrimental to orange development. Following this rationale, S lines would accumulate limonene to those maximum levels that would not compromise cell and plant viability. It may be also hypothesized that under normal growing conditions, oil glands synthesize and accumulate near-saturating concentrations of limonene (and/or its geranyl diphosphate precursor) and overexpression of limonene synthase is not able to increase further the large amount of limonene occurring in the oil glands.
Figure 1.
Molecular analyses and phenotypes of orange flavedo in sense (S) and control-treated (EV) Pineapple sweet orange plants. (A, B) Map of the T-DNA region of the binary vector used to transform S (A) and EV plants (B). LB, left T-DNA border region; RB, right T-DNA border region; nptII, gene conferring kanamycin resistance under the control of the NOS promoter and terminator regions; CitMTSE1, limonene synthase gene in sense orientation under control of the CaMV35S promoter and the NOS terminator. (C) DNA gel blot analysis indicating the loci number of the transgene. The 35S promoter was used as a probe. M: DNA molecular weight marker II from Roche Applied Science. (D) RNA gel blot analysis of total RNA extracted from flavedo of transgenic plants. RNA was separated by electrophoresis on a formaldehyde-containing agarose gel, transferred to a nylon membrane, and hybridized with a whole limonene synthase gene-specific RNA probe under stringent conditions (upper panel). Ethidium bromide staining of the same gel showing that equivalent amounts of RNA were loaded in the different lanes (lower panel). (E) The relative amounts of individual terpenes are presented as the percent (given as a fraction of unity) areas of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one. The data represent the mean values ± SEM and were derived from at least five fruits per plant.
Figure 2.
Transgenic expression of CitMTSE1 in the sense orientation in orange plants did not modify susceptibility to fungal and bacterial infections. (A– D) Evolution of the disease caused by the fungus Penicillium digitatum in mature orange fruits inoculated with 1x104 spores mL−1: percentage of infected (A) and sporulated (B) wounds in orange fruits of the EV and S lines. The results are the average ± SEM (n ≥ 20). dpi, days postinoculation. (C, D) S and EV fruits 10 d after inoculation. E to F, Evolution of the disease caused by the bacterium Xanthomonas citri subsp citri in green mature orange fruits using 106 CFU mL−1. (E) Number of wounds with symptoms in EV and S fruits. The results are the average ± SEM (n ≥ 10). (F) S and EV fruits at 4 weeks postinoculation. We repeated all experiments several times during two consecutive seasons and obtained similar results. No significant differences were found at p < 0.05 using Student’s t-test.
Our results together with the fact that in nature limonene reaches peak levels in fruit peels at the end of the growth phase, when fruits are still green but the seeds are fully developed, suggest that such high limonene doses exert an important signal effect to attract insect pests and microbial pathogens that break the peel barrier to facilitate eating of the fruit pulp by vertebrate consumers and seed dispersers.
Volatiles are important determinants of the overall aroma properties and taste of fruits.13 The compounds that are produced during the first period of fruit growth make eating by vertebrates an unpleasant experience. It is generally accepted that a primary function of secondary metabolites in immature fruits is defense from pathogens and pre-dispersal seed predators.2,4 The consumption of immature fruits would always be detrimental or repulsive because the seeds are not yet viable.4 By the end of the first period of growth, when seeds are developed, the goal is to make the fruit as appealing as possible so that seed dispersal can occur. In that phase, volatiles become an important part of the attractiveness to animals and a signal of readiness for seed dispersal.14 The identification of the limonene as a key compound in citrus fruits involved in pathogen interactions as well as insect attraction and its likely effect on seed dispersal could greatly increase our knowledge about fresh fruit trophic interactions. Just as in the pollination of flowers by insects, these interactions are complex and fine-tuned among the different organisms involved. We showed here that terpene engineering may be important for studying the basic ecological interactions between fruits, herbivores and pathogens. Determining how vertebrate dispersers fit into this tritrophic framework will provide new perspectives on these interactions.
Acknowledgments
This work was supported by grant AGL2009-08052, co-financed by FEDER and the Ministry of Science and Innovation of Spain. A.R. was supported by a PhD fellowship from the Instituto Valenciano de Investigaciones Agrarias (IVIA, Spain). We are grateful to Josep E. Peris and Clara Montesinos for his technical assistance.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/16980
References
- 1.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–43. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 2.Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am Nat. 1977;111:691–713. doi: 10.1086/283200. [DOI] [Google Scholar]
- 3.Herrera CM. Defense of ripe fruit from pests: its significance in relation to plant-disperser interactions. Am Nat. 1982;120:218–41. doi: 10.1086/283984. [DOI] [Google Scholar]
- 4.Cipollini ML, Levey DJ. Why are some fruits toxic? glycoalkaloids in Solanum and fruit choice by vertebrates. Ecology. 1997;78:782–98. [Google Scholar]
- 5.Rodríguez A, San Andrés V, Cervera M, Redondo A, Alquézar B, Shimada T, et al. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011;156:793–802. doi: 10.1104/pp.111.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada T, Endo T, Fujii H, Hara M, Ueda T, Kita M, et al. Molecular cloning and functional characterization of four monoterpene synthase genes from Citrus unshiu Marc. Plant Sci. 2004;166:49–58. doi: 10.1016/j.plantsci.2003.07.006. [DOI] [Google Scholar]
- 7.Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol. 2006;24:1441–7. doi: 10.1038/nbt1251. [DOI] [PubMed] [Google Scholar]
- 8.Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W, Verstappen FWA, et al. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell. 2003;15:2866–84. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lücker J, El Tamer MK, Schwab W, Verstappen FW, van der Plas LH, Bouwmeester HJ, et al. Monoterpene biosynthesis in lemon (Citrus limon). cDNA isolation and functional analysis of four monoterpene synthases. Eur J Biochem. 2002;269:3160–71. doi: 10.1046/j.1432-1033.2002.02985.x. [DOI] [PubMed] [Google Scholar]
- 10.Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, et al. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. doi: 10.1046/j.1365-313X.1995.08050693.x. [DOI] [Google Scholar]
- 11.Besumbes O, Sauret-Güeto S, Phillips MA, Imperial S, Rodríguez-Concepción M, Boronat A. Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of taxol. Biotechnol Bioeng. 2004;88:168–75. doi: 10.1002/bit.20237. [DOI] [PubMed] [Google Scholar]
- 12.Aharoni A, Jongsma M, Kim T, Ri M, Giri A, Verstappen F, et al. Metabolic engineering of terpenoid biosynthesis in plants. Phytochem Rev. 2006;5:49–58. doi: 10.1007/s11101-005-3747-3. [DOI] [Google Scholar]
- 13.Goff SA, Klee HJ. Plant volatile compounds: sensory cues for health and nutritional value? Science. 2006;311:815–9. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- 14.Dudareva N, Pichersky E. Metabolic engineering of plant volatiles. Curr Opin Biotechnol. 2008;19:181–9. doi: 10.1016/j.copbio.2008.02.011. [DOI] [PubMed] [Google Scholar]