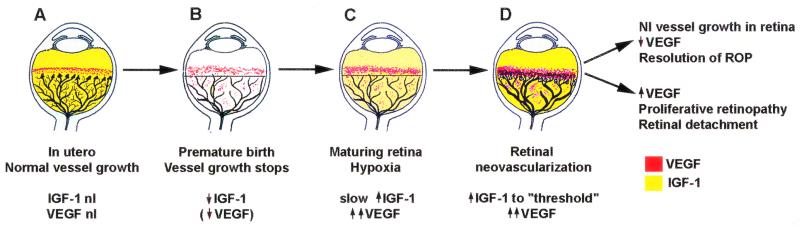
Figure 5.
Schematic representation of IGF-I/VEGF control of blood vessel development in ROP. (A) In utero, VEGF is found at the growing front of vessels. IGF-I is sufficient to allow vessel growth. (B) With premature birth, IGF-I is not maintained at in utero levels and vascular growth ceases, despite the presence of VEGF at the growing front of vessels. Both endothelial cell survival (Akt) and proliferation (mitogen-activated protein kinase) pathways are compromised. With low IGF-I and cessation of vessel growth, a demarcation line forms at the vascular front. High oxygen exposure (as occurs in animal models and in some premature infants) may also suppress VEGF, further contributing to inhibition of vessel growth. (C) As the premature infant matures, the developing but nonvascularized retina becomes hypoxic. VEGF increases in retina and vitreous. With maturation, the IGF-I level slowly increases. (D) When the IGF-I level reaches a threshold at ≈34 weeks gestation, with high VEGF levels in the vitreous, endothelial cell survival and proliferation driven by VEGF may proceed. Neovascularization ensues at the demarcation line, growing into the vitreous. If VEGF vitreal levels fall, normal retinal vessel growth can proceed. With normal vascular growth and blood flow, oxygen suppresses VEGF expression, so it will no longer be overproduced. If hypoxia (and elevated levels of VEGF) persists, further neovascularization and fibrosis leading to retinal detachment can occur.