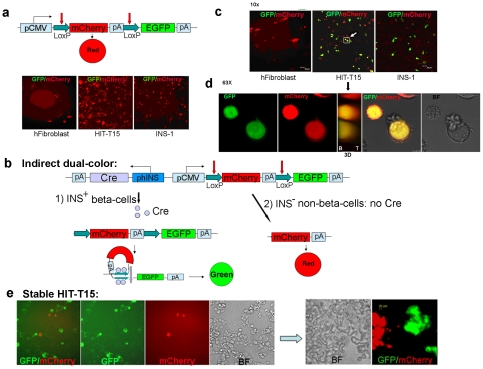
Figure 3. “Indirect" dual-color design of the reporter construct.
(a) Transfection of cells with CMV-L-mCherry-L-EGFP. Without Cre recombinase in the construct, all cells exhibited red fluorescence. (b) Schematic and anticipated behavior of pA-Cre-phIns-CMV-L-mCherry-L-EGFP. INS+ beta-cells transfected by this plasmid should activate Cre recombinase expression and excise the mCherry reporter by Cre-LoxP recombination, thus converting red fluorescence to green. In contrast, transfected INS− non-beta-cells should only exhibit red fluorescence. (c) After transfection of cells with pA-Cre-phIns-CMV-L-mCherry-L-EGFP, HIT-T15 and INS-1 expressed EGFP, indicating that mCherry was excised by Cre recombinase expressed under the human insulin promoter. This demonstrates the reporter's specificity to produce EGFP in only INS+ cells. (d) Highly magnified (63×) projection images of two yellow cells marked as the white square with a white arrow in Fig. 2c from Z-stacks (10×), illustrating both red and green fluorescence. In the middle panel, the 3D picture shows co-localization of both colors (B, bottom; T, top). (e) Stable HIT-T15 cell population containing the “indirect" dual-color construct (Neor). ∼70% of the stably transfected cells were green, indicating mCherry excision. Another ∼10% were red, indicating the absence of mCherry excision and suggesting that the insulin promoter was never activated. The remaining ∼20% of cells exhibited no fluorescence, suggesting transcriptional inactivation of the CMV promoter. The right panel depicts the cells after more than 6 days in culture, compared to cells at 1 day after splitting shown in the left panel. No yellow fluorescent cells were observed. These results suggest that at least two phenotypically heterogeneous cells arise in the HIT-T15 cell culture model.