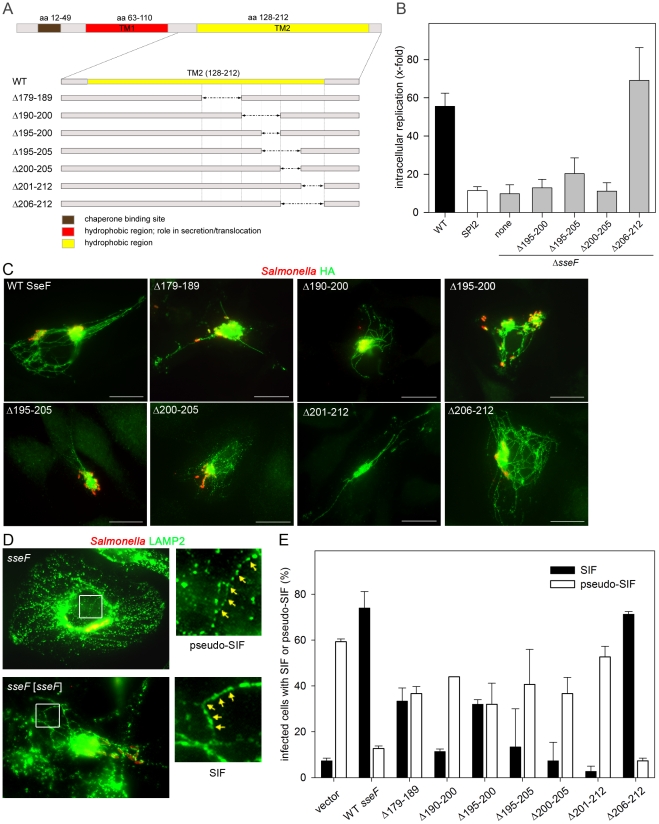
Figure 1. Functional dissection of the C-terminal hydrophobic domain of SseF.
A) Location of in-frame deletions of various extents in the C-terminal moiety of SseF. Functions of domains in SseF as revealed by previous studies [19], [34] are indicated. B) Analyses of intracellular replication in HeLa cells of Salmonella WT, a SPI2 null mutant strain, and the sseF strain without or with plasmids for the expression of various mutant alleles of sseF. The amount of intracellular colony-forming units (CFU) was determined 2 h and 16 h after infection and intracellular replication is the ratio of CFU at 16 h/2 h. Means and standard deviation of three assays are shown. C) Translocation of deletion variants of SseF. HeLa cells were infected with the sseF strain harboring plasmids for the expression of sseF::HA or various mutant alleles of sseF as indicated. Cells were fixed 16 h p.i. and immuno-stained for SseF-HA (detected with α rat Alexa488, green) and LPS (detected with α rabbit Alexa568, red). Scale bars: 20 µm. D, E) SIF phenotypes in cells after translation of various SseF variants. HeLa cells were infected with the sseF strain without plasmid or with plasmids for the expression of WT sseF or various mutant alleles as indicated. Cells were fixed 16 h after infection and immuno-stained for Salmonella LPS (red) and LAMP2 (green). The formation of SIF or pseudo-SIF in infected host cells was scored. D) Representative cells showing SIF or pseudo-SIF formation are shown. The white frame was enlarged and arrows indicate the typical appearance of SIF and pseudo-SIF. E) Quantification of SIF and pseudo-SIF formation by the various deletion strains. At least 50 infected cells were identified and the percentage of cells showing SIF (filled bars) or pseudo-SIF (open bars) was calculated. The means and standard deviations of three independent experiments are shown.