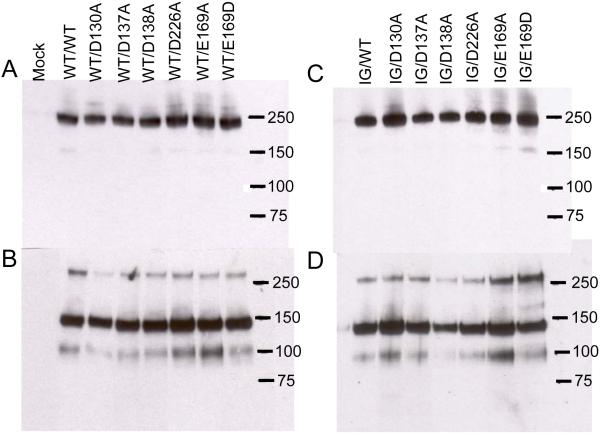
FIGURE 1. Western blotting of recombinant TRα2β1-Fc integrins.
Recombinant integrins were partially purified from cell culture supernatants using protein A Sepharose. Aliquots of purified proteins were run on 3-8 % SDS-PAGE gels under non-reducing (panels A, B) or reducing conditions (panels C, D), transferred to nitrocellulose and blotted with anti-human Fc conjugated with horseradish peroxidase. Panels A and C, culture supernatants from cells transfected with wild-type (WT) TRα2 and WT or cation-binding site mutant TRβ1. Panels B and D, culture supernatants from cells transfected with I332G mutant TRα2 (IG) and WT or cation-binding site mutant TRβ1. Migration positions of molecular weight markers are indicated (kDa). Under non-reducing conditions a predominant band at ~ 240 kDa is observed, close to the expected size for the TRα2β1-Fc heterodimer. Under reducing conditions disulfide bonds between the Fc hinge regions are broken and two predominant bands of ~ 140 kDa and ~ 100 kDa are observed, corresponding to the expected sizes of TRα2 and TRβ1 proteins, respectively. For unknown reasons the β subunit band reacts much more weakly with the anti-human Fc antibody than the α subunit band (31). The 240 and 100 kDa bands are reactive with the anti-β1 mAb TS2/16; the 240 and 140 kDa bands are reactive with anti-α2 mAb JA212 (not shown). These bands are not observed in proteins purified from the supernatants of mock-transfected cells.