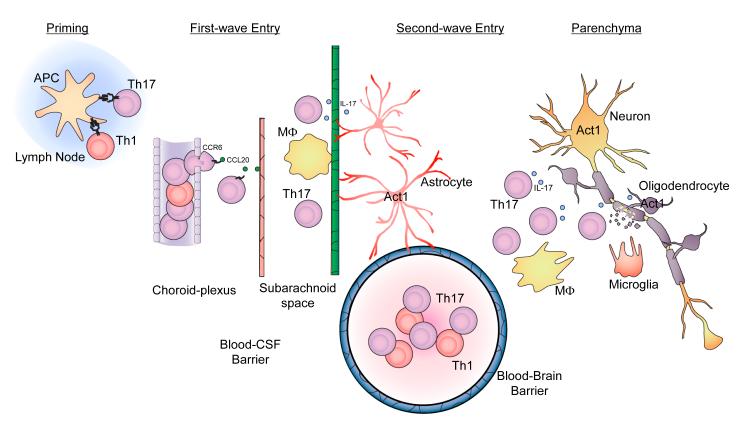
Figure 3. Th17 cells in the initiation and effector stages of EAE.
(A) T cell priming and activation. Protein antigens (myelin components) and peptides are presented by Ag-presenting cells (APCs) within secondary lymphoid organs to the neuroantigen-reactive T cells leading to Th1 and Th17 cell activation and expansion. (B) First wave of Th17 cell entry. After priming in peripheral lymph nodes, CCR6+ Th17 cells escape from choroid-plexus blood vessels, migrate towards choroid-plexus epithelial cells, which express CCL20 (ligand for CCR6). Restimulation of T cells by macrophages in the subarachniod space leads to expansion of antigen-specific Th17 cells. In the first wave, Th17 cells cross the blood-CSF barrier. (C) Second wave of Th17 cell entry hypothesized in the proposal. At the onset of Wave 2, after activation of the blood-brain barrier, Th17 cells adhere at the endothelium via upregulated adhesion molecules and begin penetration of the capillary endothelium. Once the activated lymphocytes have extravasated, they are then re-activated by their cognate antigens presented by local APCs in the CNS, leading to the amplification of the inflammatory cascade in the CNS. The large numbers of activated Th17 cells and other inflammatory cells subsequently migrate deeper into the white matter of the CNS parenchyma, resulting in tissue destruction including demyelination and eventually neurologic deficit.