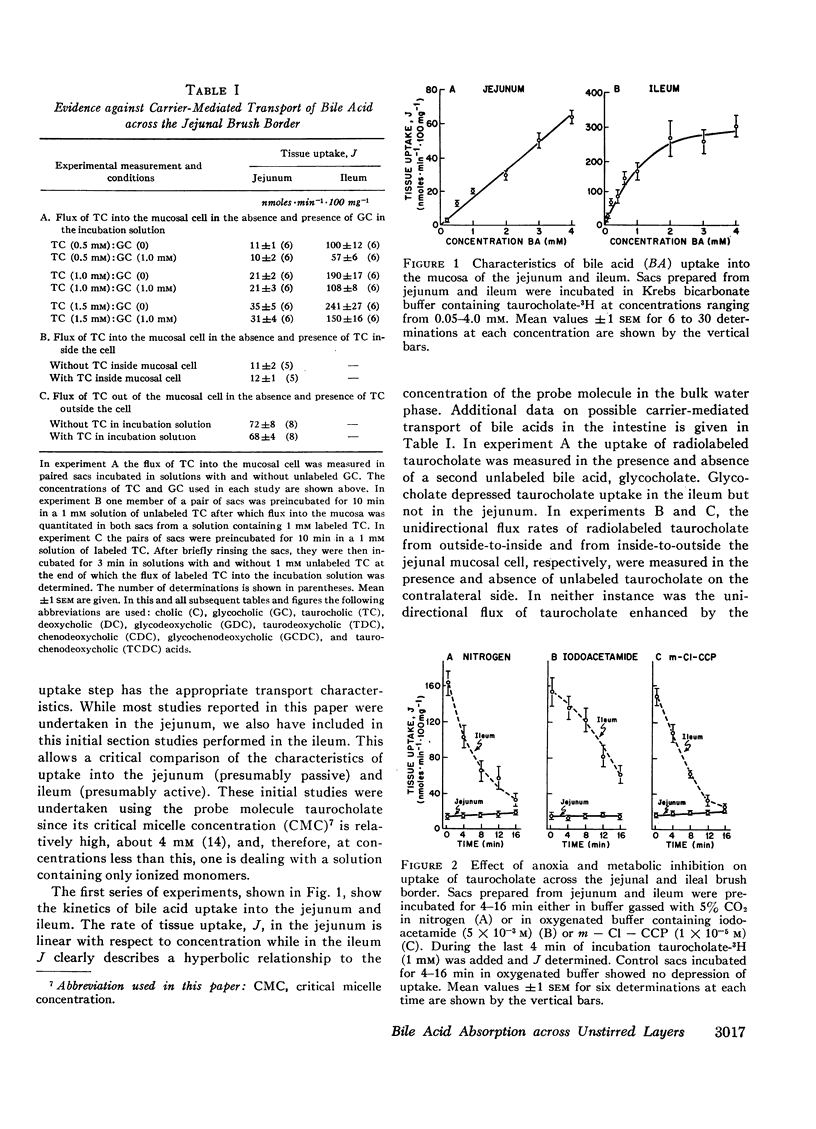
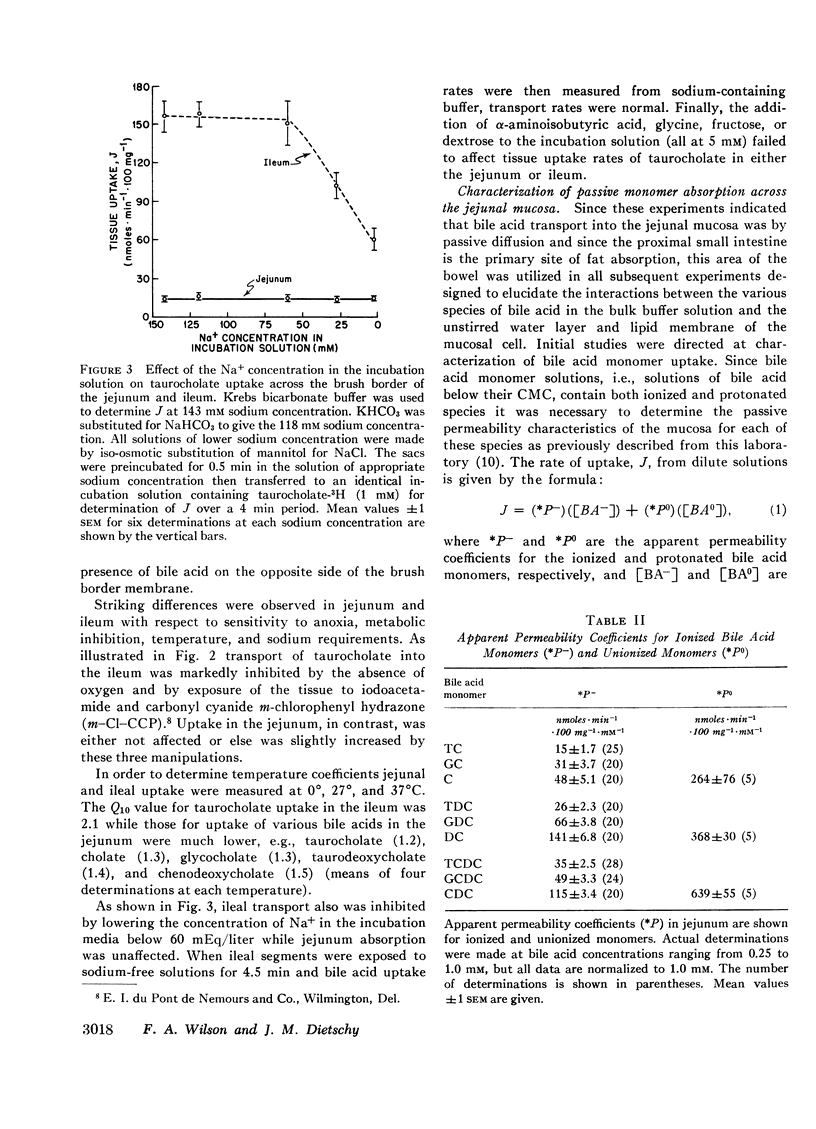
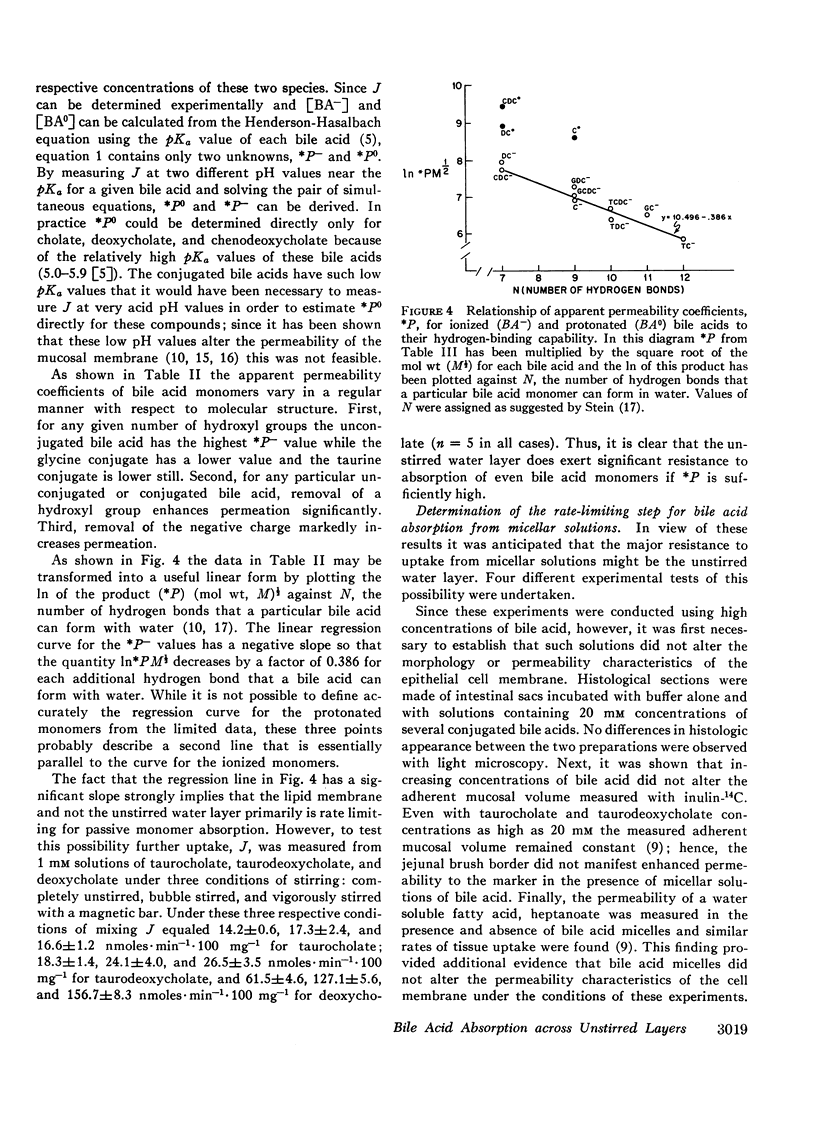
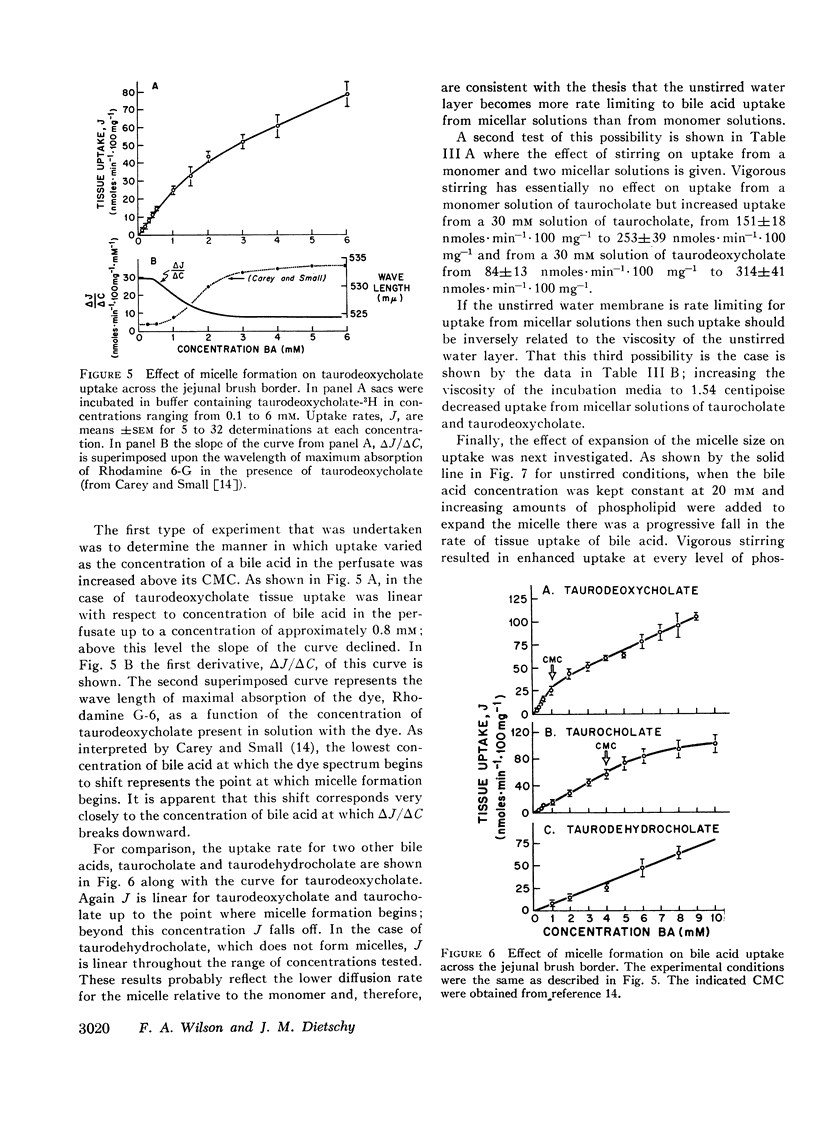
Abstract
We have examined the rate-limiting steps involved in bile acid absorption across the unstirred water layer and lipid cell membrane of the jejunal mucosa. Uptake of the polar bile acid taurocholate is limited solely by the cell membrane since this compound permeates the unstirred water layer more rapidly than the lipid cell membrane and stirring does not enhance uptake. With less polar bile acids which permeate the cell membrane relatively more rapidly, however, the unstirred water layer does exert resistance to mucosal uptake of these compounds. That the unstirred water layer is even more rate limiting to uptake from micellar solutions is indicated by the facts that the rate of bile acid absorption from such solutions is lower than from corresponding monomer solutions, stirring markedly enhances uptake from micellar solutions while increases in viscosity of the incubation media depress uptake and expansion of the micelle size further depresses absorption rates. We also have examined the important question of whether the micelle crosses the brush border intact once it reaches the aqueous-lipid interface. The observations that the calculated permeation rate of the micelle should be extremely low, the rate of mucosal cell uptake plateaus at a constant value when the critical micelle concentration is reached at the aqueous-lipid interface, and the different components of a mixed micelle are taken up at different rates indicate that uptake of the intact micelle does not occur; rather, bile acid absorption must be explained in terms of monomers in equilibrium with the micelle. Finally, after correction of the permeability coefficients of the various bile acids for the unstirred layer resistance the incremental partial molar free energy of solution of the hydroxyl group in the brush border membrane was calculated to equal −6126 cal·mole−1 indicating that passive diffusion of these compounds occurs through a very polar region of the cell membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey M. C., Small D. M. Micellar properties of dihydroxy and trihydroxy bile salts: effects of counterion and temperature. J Colloid Interface Sci. 1969 Nov;31(3):382–396. doi: 10.1016/0021-9797(69)90181-7. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968 May;9(3):297–309. [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- Isselbacher K. J. Biochemical aspects of lipid malabsorption. Fed Proc. 1967 Sep;26(5):1420–1425. [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: role in triglyceride and cholesterol transport during fat absorption. J Clin Invest. 1969 Dec;48(12):2367–2373. doi: 10.1172/JCI106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H., LEAF A., SCHWARTZ W. B. DIFFUSION OF WEAK ACIDS ACROSS THE TOAD BLADDER. INFLUENCE OF PH ON NON-IONIC PERMEABILITY COEFFICIENTS. J Gen Physiol. 1964 Nov;48:379–389. doi: 10.1085/jgp.48.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee V. L., Wilson F. A., Dietschy J. M. Determination of unidirectional uptake rates for lipids across the intestinal brush border. J Lipid Res. 1972 Mar;13(2):184–192. [PubMed] [Google Scholar]
- Schiff E. R., Small N. C., Dietschy J. M. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972 Jun;51(6):1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Differential diagnostic approach to clinical problems of malabsorption. Gastroenterology. 1971 Dec;61(6):911–931. [PubMed] [Google Scholar]
- Wilson F. A., Sallee V. L., Dietschy J. M. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science. 1971 Dec 3;174(4013):1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]
- Woodford F. P. Enlargement of taurocholate micelles by added cholesterol and monoolein: self-diffusion measurements. J Lipid Res. 1969 Sep;10(5):539–545. [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Patterns of non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):227–271. doi: 10.1098/rspb.1969.0021. [DOI] [PubMed] [Google Scholar]


