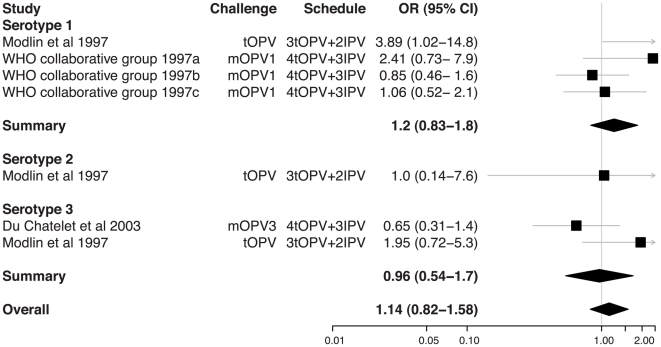
Figure 5. Relative odds of shedding vaccine poliovirus after challenge among individuals vaccinated with IPV in addition to OPV compared with individuals vaccinated with OPV only.
Labeling as for Figure 2. The schedule indicates the number and type of OPV doses received by both groups and the number of doses of IPV that were added in the intervention group. In two studies, IPV was administered simultaneously with OPV at 6, 10, and 14 weeks (Modlin et al. 1997 [47] and du Chatelet et al. 2003 [48]), and in one study IPV was administered before and at the same time as OPV (schedule was IPV, IPV/OPV, OPV, OPV at 2, 4, 6, 15 months; WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines 1997 [49]). The χ2 test for heterogeneity among studies for serotypes 1 and 3 was not significant for each serotype (p-values 0.13 and 0.08) or for the serotypes combined (p-value 0.14).