Table 1.
Optimization of the catalytic system.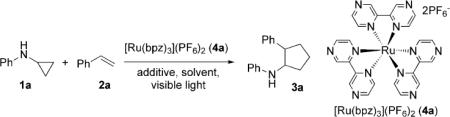
| Entry | Conditions[a] | t [h] | Conv. of 1a [%][b] | Yield of 3a [%][b] |
|---|---|---|---|---|
| 1 | 4a (2 mol%), Air, CH3NO2 | 12 | 100 | 21 |
| 2 | 4a (2 mol%), CH3NO2 | 3 | 100 | 96 |
| 3 | without 4a, CH3NO2 | 12 | 25 | 16 |
| 4 | 4a (2 mol%), CH3NO2, lightbulb off | 12 | 35 | 9 |
| 5 | 4b (2 mol%), CH3NO2 | 12 | 100 | 79 |
| 6 | 4c (2 mol%), CH3NO2 | 12 | 100 | 73 |
Reaction conditions: 1a (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), irradiation with a 13 W fluorescent lightbulb at RT.
Measured by GC using dodecane as an internal standard. bpz=2, 2′-bipyrazine.
