Table 2.
[3+2] Cycloaddition of styrene (2a) with monocyclic cyclopropylamines (1).[a]
| Entry | Substrate | Product | t [h] | Yield [%][d] |
|---|---|---|---|---|
| 1[b] | 1a, R=H | 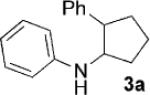 |
3 | 87 |
| 2[b] | 1b, R=4-CI | 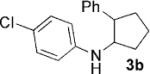 |
6 | 82 |
| 3[b] | 1c, R=4-CF3 | 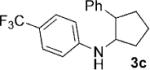 |
4 | 82 |
| 4[c] | 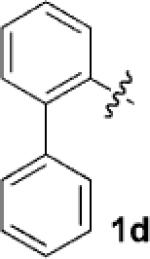 |
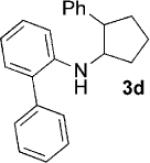 |
24 | 80 |
| 5[c] | 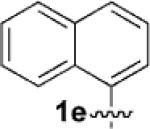 |
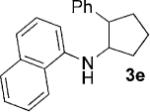 |
6 | 75 |
| 6[b] | 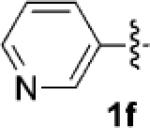 |
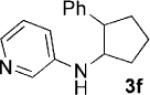 |
48 | 71 |
Reaction conditions: substrate (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), 4a (2 mol%), irradiation with a 13 W fluorescent lightbulb at RT.
d.r.=1:1
d.r.=3:2 as determined by 1H NMR spectroscopy of crude products.
Yield of the combined isomers after isolation.
