Table 3.
[3+2] Cycloaddition ofstyrene (2a) with bicyclic cyclopropylamines 5.[a]
| Entry | Substrate | Product[b] | t [h] | Yield [%][c] |
|---|---|---|---|---|
| d.r.[d] | ||||
| 1 | 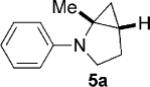 |
 |
5 | 77 4:1 |
| 2 | 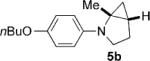 |
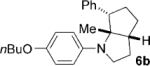 |
8 | 74 5:1 |
| 3 | 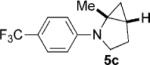 |
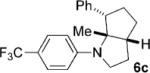 |
36 | 69 5:1 |
| 4 | 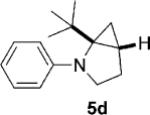 |
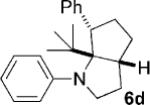 |
12 | 28 (64)[e] >25:1 |
| 5 | 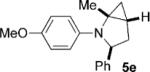 |
 |
6 | 72 3:1 |
| 6 |  |
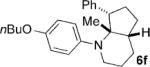 |
12 | 58 4:1 |
Reaction conditions: 5a–f (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), 4a (2 mol%), irradiation with a 13 W fluorescent lightbulb at RT.
Only the major diastereoisomer shown.
Combined yields of the two isomers after chromatography.
Determined by 1H NMR analysis of the crude products (α/β).
Based on recovered 5d.
