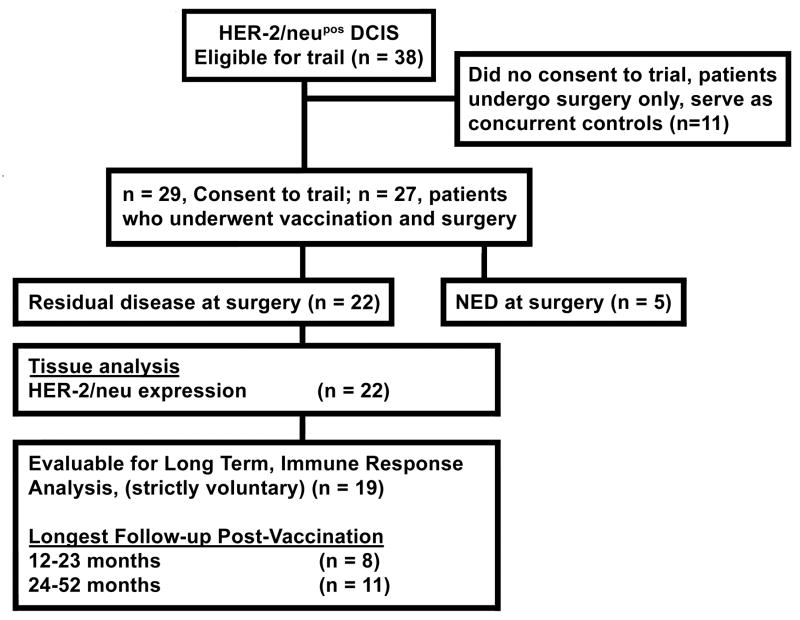
FIGURE 1.
A flow diagram outlining the patient population participating in the clinical trial. Of the 38 patients eligible for the trial, 27 patients gave written informed consent and enrolled on the IRB-approved trial. Five patients had no residual disease following vaccination while 22 patients had residual disease thus allowing for analysis of post-vaccine immune response.