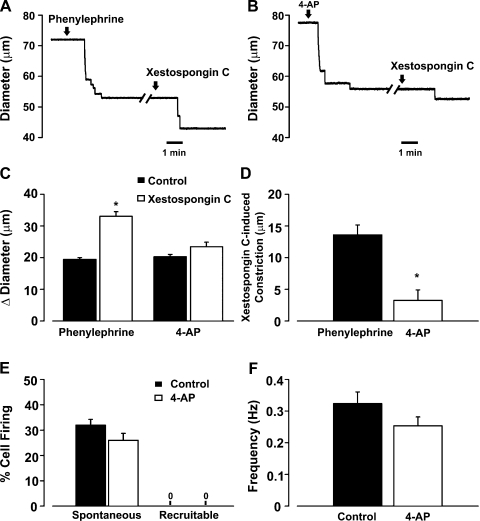
Fig. 11.
IP3 flux in the initiation of myoendothelial feedback and recruitable Ca2+ wavelets. A–D: feed arteries were isolated, pressurized, and superfused with phenylephrine (0.1 μM, an α-adrenoreceptor agonist, n = 6) or 4-AP (2 mM, a voltage-dependent K+ channel inhibitor, n = 6), while diameter changes were monitored in the presence or absence of luminal xestospongin C (10 μM, an IP3R inhibitor). A and B: representative traces of xestospongin C-induced constriction in feed arteries pretreated with phenylephrine or 4-AP. C and D: summary data highlighting arterial responsiveness to phenylephrine (n = 6) or 4-AP (n = 6) alone and in combination with xestospongin C. Diameter at rest, maximum diameter, and minimum diameter were 93 ± 5, 110 ± 3, and 20 ± 1 μm (phenylephrine experiment) and 73 ± 3, 87 ± 5, and 20 ± 1 μm (4-AP experiment). *Significantly different from control (C) or phenylephrine (D). Diameter change due to luminal perfusion of xestospongin C alone was 3.3 ± 1.6 μm. E and F: feed arteries were opened, pinned to a Sylgard block, and loaded with fluo 4-AM. Ca2+ wavelets were monitored in the absence and presence of 4-AP. See Fig. 8 legend for analysis details. Summary data (n = 50 cells from 5 arteries) highlight the inability of 4-AP to alter generation of spontaneous and recruitable Ca2+ wavelets.