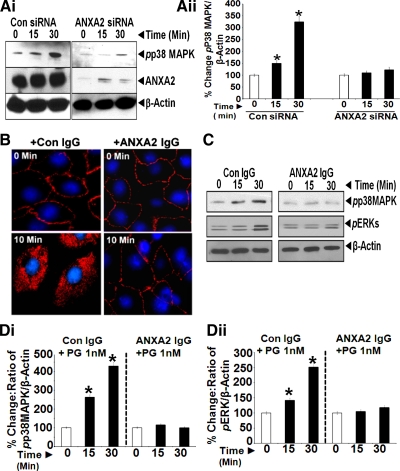
Fig. 4.
A: ANXA2 expression is required for measuring activation of p38 MAPK in response to PG. IEC-18 cells, treated with either control or ANXA2 targeting siRNA, were stimulated with 1 nM PG from 0–30 min and processed by WIB for phosphorylated (p)p38MAPK and ANXA2. β-Actin levels were used as a loading control. Representative blot from two experiments are presented in Ai. Ratio of pp38MAPK-to-β-actin in cells treated with either control or ANXA2 siRNA are presented in Aii. The data in Aii are means ± SE of three blots from two experiments. *Significant difference vs. 0-min values (P < 0.05). B: freely available CS-ANXA2 is required for internalization of PG in the cells. IEC-18 cells in culture on glass slides were pretreated with either control (preimmune) IgG or anti-ANXA2-IgG (1:200) at equivalent concentrations as previously described (48, 60). Cells were pretreated with the antibodies for 30 min at room temperature, followed by stimulation with 10 nM PG for either 0 or 10 min. Cells thus treated were processed for epifluorescence microscopy. Representative images obtained at ×20 from two experiments were computer enhanced and are presented in B. Cells treated with either control or anti-ANXA2-IgG demonstrated the presence of PG associated with the cellular membranes. However, after 10 min of PG stimulation, internalization of PG was observed only in cells treated with control IgG, whereas cells treated with anti-ANXA2 IgG demonstrated the presence of PG on cellular membranes only, with no evidence of internalization of PG. Since anti-ANXA2-IgG likely binds CS-ANXA2, the absence of internalization of PG in cells treated with anti-ANXA2 antibodies suggests that freely available CS-ANXA2 is required for mediating internalization of PG in IEC-18 cells. C and D: internalization of PG is required for activation of p38MAPK/ERKs. IEC-18 cells were pretreated with either control IgG or anti-ANXA2-IgG as described above, followed by stimulation with 1 nM PG for 0–30 min, and the cells processed by WIB for phosphorylated (p) p38MAPK/ERKs; β-actin levels were used as a loading control. Representative blots from two experiments are presented in C. Ratio of target protein-to-β-actin, as described in text, is shown in Di (pp38MAPK) and Dii (pERK). Data in D are means ± SE of four blots from two experiments. *Significant difference vs. 0-min values (P < 0.05).