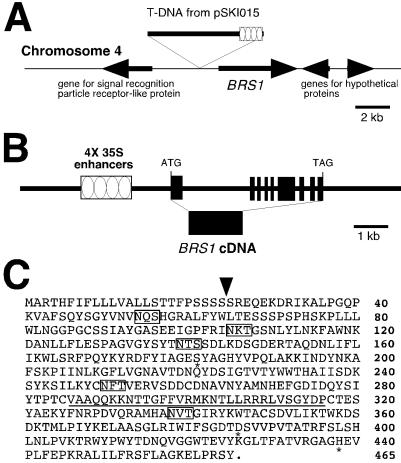
Figure 2.
The BRS1 gene encodes a protein with homology to serine carboxypeptidases (54% and 53% identity with wheat and barley serine carboxypeptidase II proteins; 28% identity with yeast Kex1p protein). (A) The flanking sequence of the T-DNA was cloned via inverse PCR. The T-DNA insert localizes to the bottom part of chromosome IV. 5′ of the T-DNA, 6.5 kb from the 4 × 35S enhancers, there is a gene encoding a signal recognition particle receptor-like protein. 3′ of the T-DNA, 1.1 kb from 4 × 35S enhancers, there is a gene encoding a serine carboxypeptidase, which was subsequently confirmed as the suppressor, BRS1. (B) Comparison of the cDNA and genomic sequences indicated that BRS1 has 9 exons and 8 introns. (C) Deduced amino acid sequence of BRS1. A possible signal peptide cleavage site is indicated by an arrow. Five potential N-linked glycosylation sites are marked in the open boxes. The asterisks below an amino acid indicate the three putative “catalytic triad” amino acids, S181, D386, and H438. A possible cleavage linker peptide is underlined. The BRS1 sequence was obtained from GenBank (accession no. AL161577, reference GI: 7269962).