Abstract
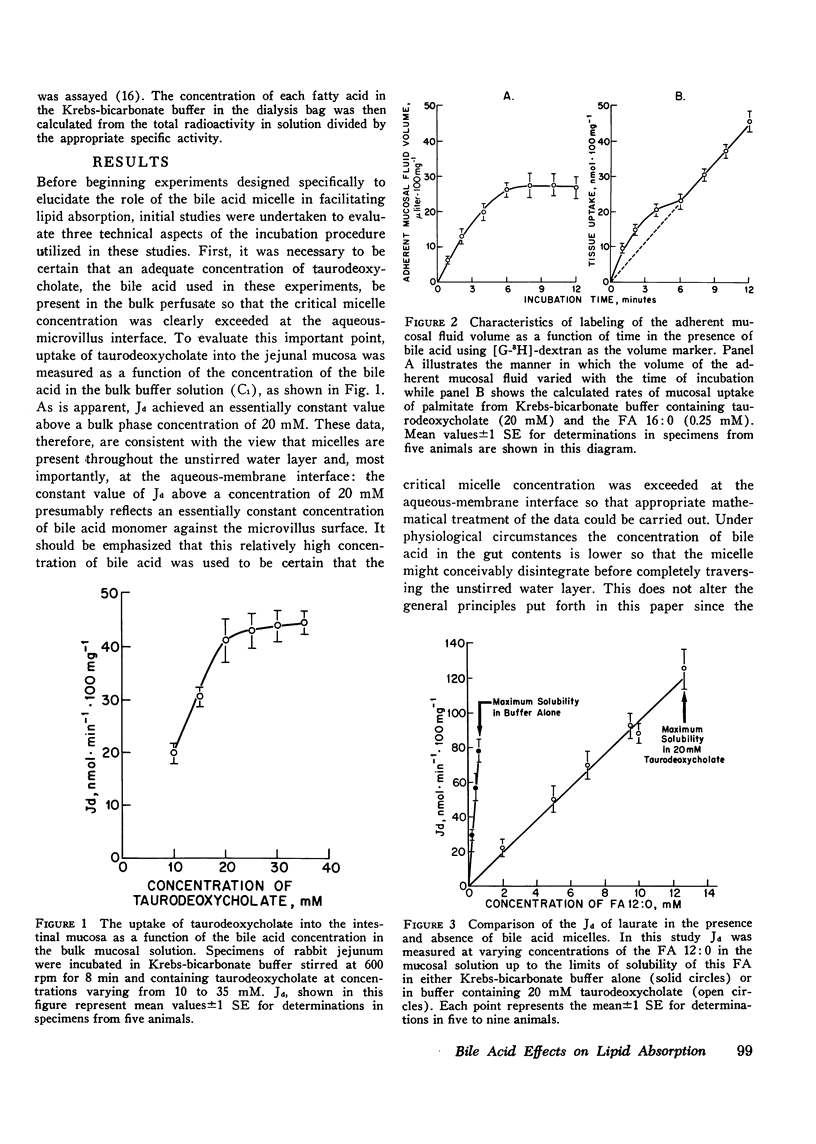
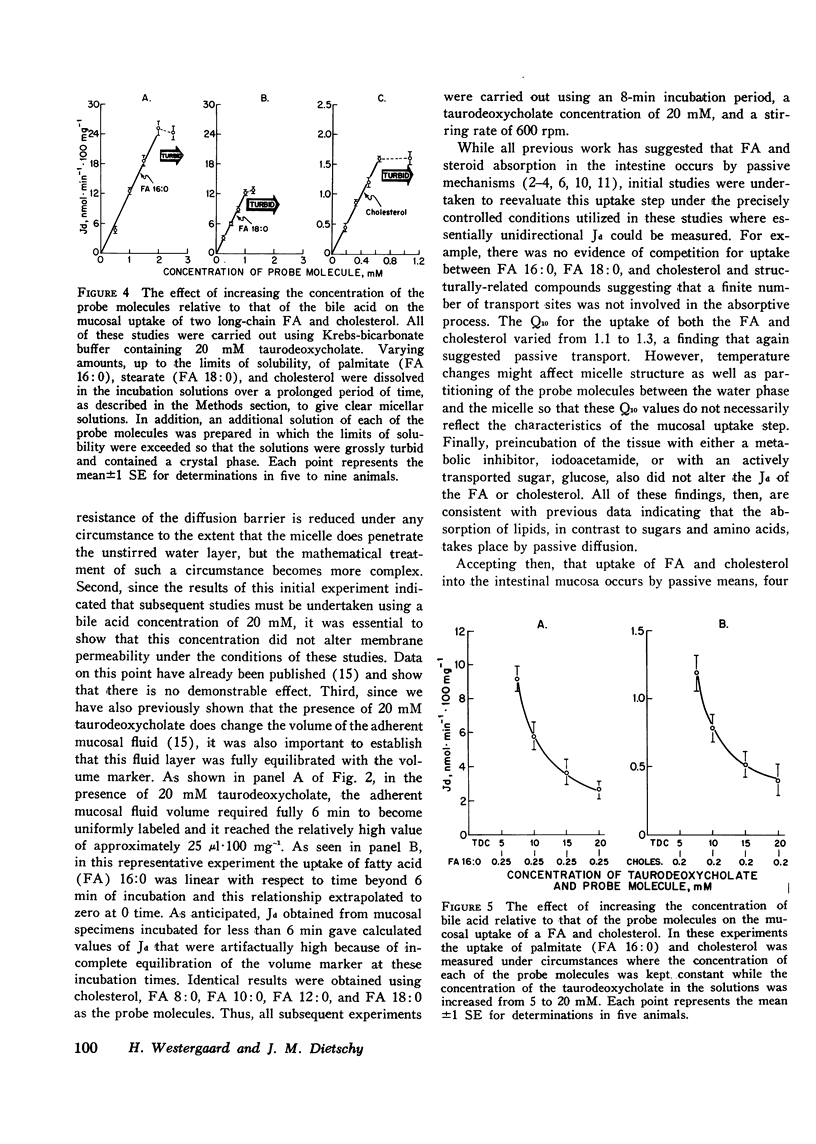
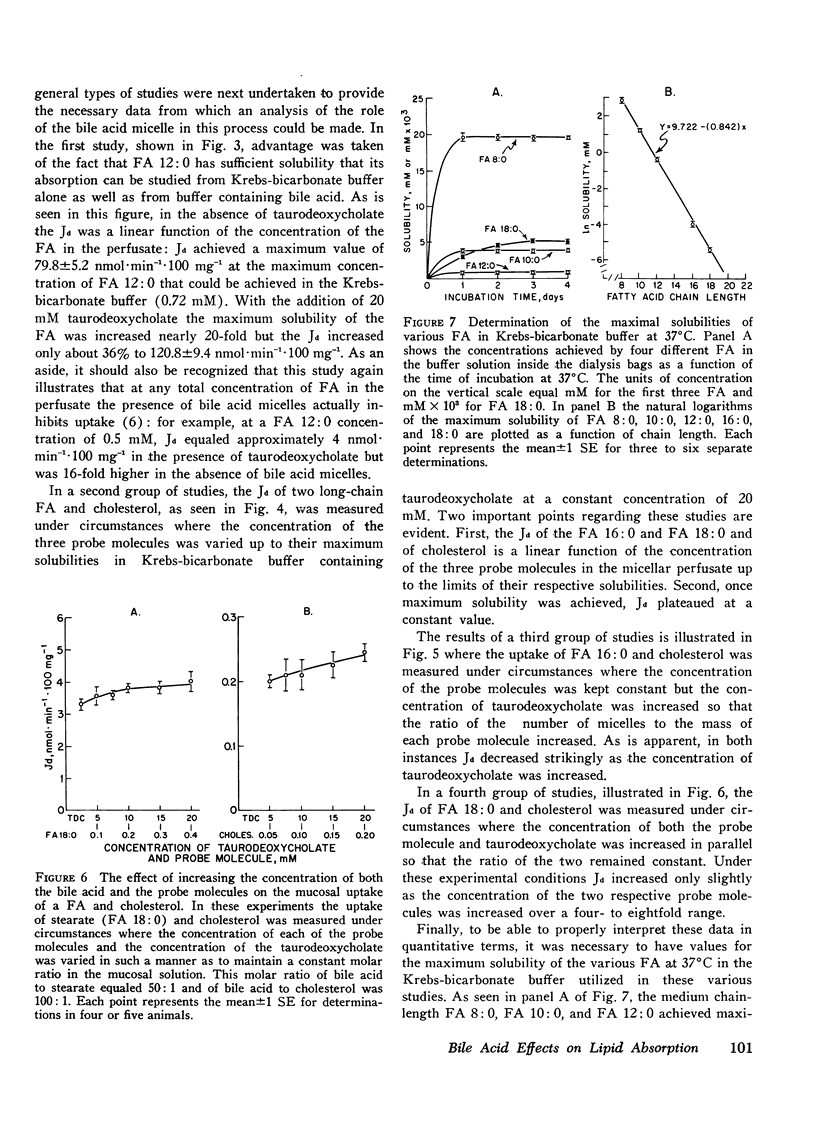
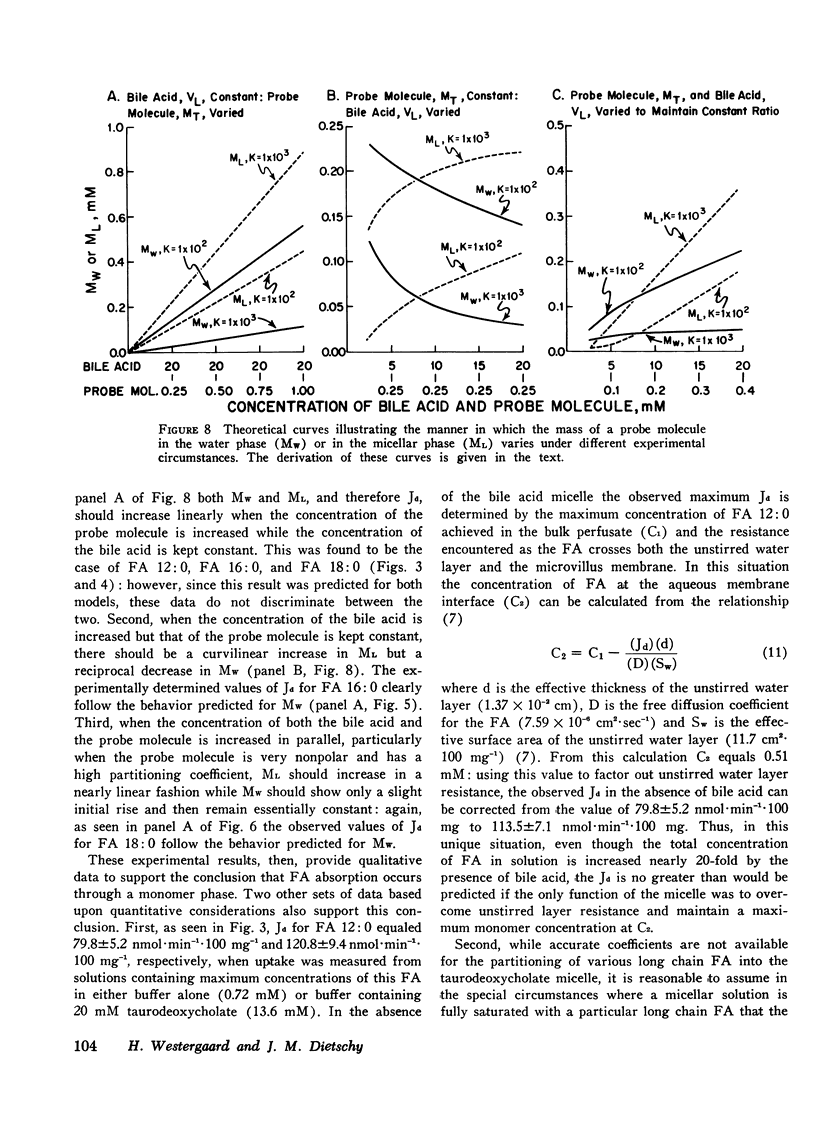
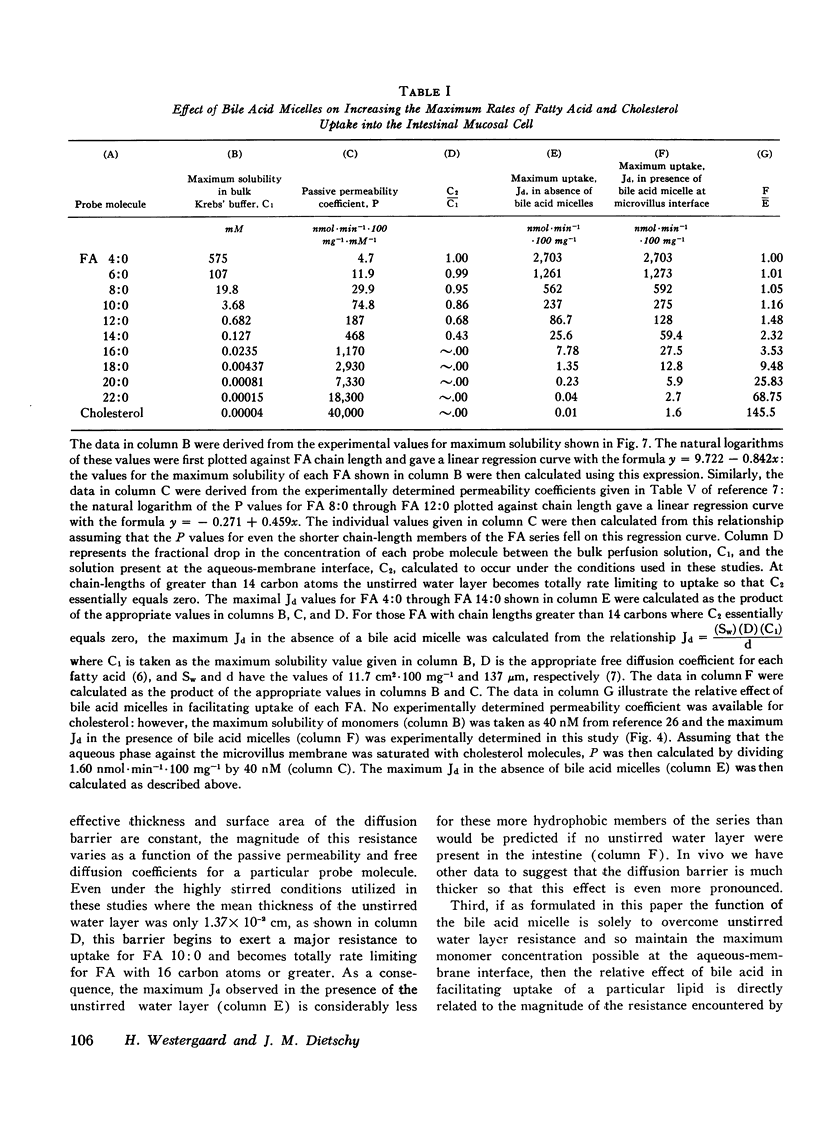
Studies were undertaken to define the mechanism whereby bile acid facilitates fatty acid and cholesterol uptake into the intestinal mucosal cell. Initial studies showed that the rate of uptake (Jd) of several fatty acids and cholesterol was a linear function of the concentration of these molecules in the bulk phase if the concentration of bile acid was kept constant. In contrast, Jd decreased markedly when the concentration of bile acid was increased relative to that of the probe molecule but remained essentially constant when the concentration of both the bile acid and probe molecule was increased in parallel. In other studies Jd for lauric acid measured from solutions containing either 0 or 20 mM taurodeoxycholate and saturated with the fatty acid equaled 79.8+/-5.2 and 120.8+/-9.4 nmol.min(-1).100 mg(-1), respectively: after correction for unstirred layer resistance, however, the former value equaled 113.5+/-7.1 nmol.min(-1).100 mg(-1). Maximum values of Jd for the saturated fatty acids with 12, 16, and 18 carbons equaled 120.8+/-9.4, 24.1+/-3.2, and 13.6+/-1.1 nmol.min(-1).100 mg(-1), respectively. These values essentially equaled those derived by multiplying the maximum solubility times the passive permeability coefficients appropriate for each of these compounds. The theoretical equations were then derived that define the expected behavior of Jd for the various lipids under these different experimental circumstances where the mechanism of absorption was assumed to occur either by uptake of the whole micelle, during interaction of the micelle with an infinite number of sites on the microvillus membrane or through a monomer phase of lipid molecules in equilibrium with the micelle. The experimental results were consistent both qualitatively and quantitatively with the third model indicating that the principle role of the micelle in facilitating lipid absorption is to overcome unstirred layer resistance while the actual process of fatty acid and cholesterol absorption occurs through a monomer phase in equilibrium with the micelle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey M. C., Small D. M. Micellar properties of dihydroxy and trihydroxy bile salts: effects of counterion and temperature. J Colloid Interface Sci. 1969 Nov;31(3):382–396. doi: 10.1016/0021-9797(69)90181-7. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- FERNANDES J., van de KAMER J. H., WEIJERS H. A. Differences in absorption of the various fatty acids studied in children with steatorrhea. J Clin Invest. 1962 Mar;41:488–494. doi: 10.1172/JCI104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZER A. C. Fate of dietary fat in the body. Nature. 1962 Jun 9;194:908–909. doi: 10.1038/194908a0. [DOI] [PubMed] [Google Scholar]
- Gregg J. A. New solvent systems for thin-layer chromatography of bile acids. J Lipid Res. 1966 Jul;7(4):579–581. [PubMed] [Google Scholar]
- Haberland M. E., Reynolds J. A. Self-association of cholesterol in aqueous solution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2313–2316. doi: 10.1073/pnas.70.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E. The relationship between uptake in vitro of oleic acid and micellar solubilization. Biochim Biophys Acta. 1970;196(2):193–203. doi: 10.1016/0005-2736(70)90006-4. [DOI] [PubMed] [Google Scholar]
- Lukie B. E., Westergaard H., Dietschy J. M. Validation of a chamber that allows measurement of both tissue uptake rates and unstirred layer thicknesses in the intestine under conditions of controlled stirring. Gastroenterology. 1974 Oct;67(4):652–661. [PubMed] [Google Scholar]
- Porter H. P., Saunders D. R., Tytgat G., Brunser O., Rubin C. E. Fat absorption in bile fistula man. A morphological and biochemical study. Gastroenterology. 1971 Jun;60(6):1008–1019. [PubMed] [Google Scholar]
- SANDERS E., ASHWORTH C. T. A study of particulate intestinal absorption and hepatocellular uptake. Use of polystyrene latex particles. Exp Cell Res. 1961 Jan;22:137–145. doi: 10.1016/0014-4827(61)90092-1. [DOI] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., CHAIKOFF I. L., REINHARDT W. O. C14-Cholesterol. V. Obligatory function of bile in intestinal absorption of cholesterol. J Biol Chem. 1952 Sep;198(1):111–114. [PubMed] [Google Scholar]
- Sallee V. L. Apparent monomer activity of saturated fatty acids im micellar bile salt solutions measured by a polyethylene partitioning system. J Lipid Res. 1974 Jan;15(1):56–64. [PubMed] [Google Scholar]
- Sallee V. L., Dietschy J. M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973 Jul;14(4):475–484. [PubMed] [Google Scholar]
- Sallee V. L., Wilson F. A., Dietschy J. M. Determination of unidirectional uptake rates for lipids across the intestinal brush border. J Lipid Res. 1972 Mar;13(2):184–192. [PubMed] [Google Scholar]
- Sherill B. C., Dietschy J. M. Permeability characteristics of the adipocyte cell membrane and partitioning characteristics of the adipocyte triglyceride core. J Membr Biol. 1975;23(3-4):367–383. doi: 10.1007/BF01870258. [DOI] [PubMed] [Google Scholar]
- Simmonds W. J. The role of micellar solubilization in lipid absorption. Aust J Exp Biol Med Sci. 1972 Aug;50(4):403–421. doi: 10.1038/icb.1972.35. [DOI] [PubMed] [Google Scholar]
- Simpson R. B., Ashbrook J. D., Santos E. C., Spector A. A. Partition of fatty acids. J Lipid Res. 1974 Jul;15(4):415–422. [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest. 1972 Dec;51(12):3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Differential diagnostic approach to clinical problems of malabsorption. Gastroenterology. 1971 Dec;61(6):911–931. [PubMed] [Google Scholar]


