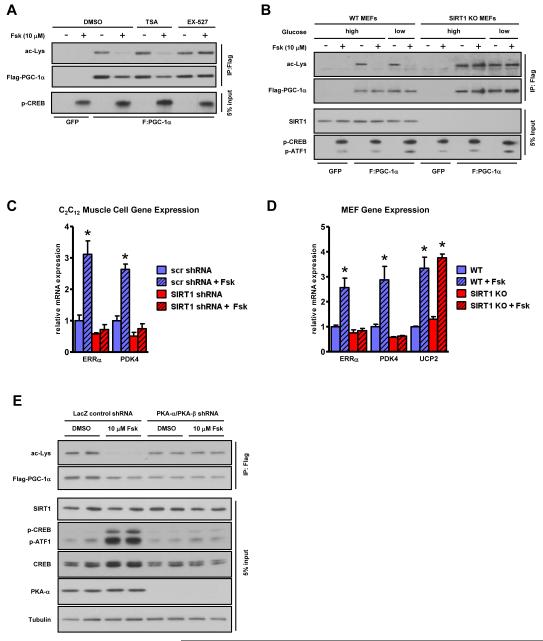
Figure 2. cAMP-mediated PGC-1α deacetylation/activation is SIRT1 and PKA-dependent.
A, Pharmacological inhibition and, B, genetic ablation of SIRT1 abrogates cAMP-mediated PGC-1α deacetylation. MEFs were subject to cAMP agonism under conditions of high (25 mM) and low (2.5 mM) glucose. C, SIRT1 knockdown by shRNA in skeletal muscle cells or, D, genetic ablation in fibroblasts abolishes the forskolin-mediated increase in fatty acid oxidative genes. PGC-1α was adenovirally expressed in differentiated C2C12 myotubes in addition to either scrambled (scr) control or SIRT1 shRNA for 72 hours to ensure knockdown. Skeletal muscle cells and MEFs were treated with Fsk for 6 h before RNA isolation and mRNA quantification; statistical significance was assessed using one-way ANOVA with a Tukey post-test, *, p<0.01, DMSO vs Fsk. E, cAMP-mediated PGC-1α deacetylation is PKA dependent. PGC-1α was immunoprecipitated from DMSO or Fsk-treated U2OS cell lines expressing either control LacZ shRNA or shRNAs against both PKA-α and β catalytic subunits (Figure S2D). Data are presented as means ± S.D.