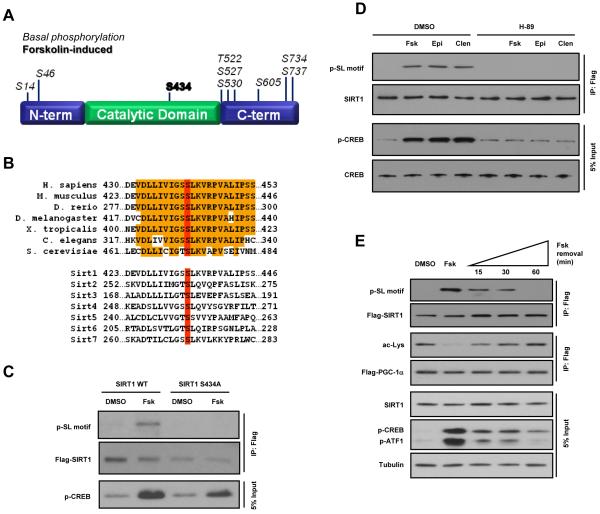
Figure 3. PKA activation results in phosphorylation of SIRT1 on serine 434.
A, Schematic depicting phosphorylation sites detected using mass spectrometric analysis of SIRT1 protein immunoprecipitated from U2OS cells incubated with Fsk or DMSO. Murine SIRT1 residue, S434 (bold), was found to be differentially phosphorylated following stimulation of the cAMP signaling pathway. B, Phylogenetic alignment shows complete conservation of SIRT1 S434 (red) among orthologs as well as other mammalian Sirtuin family members. C, Mutation of S434 abolishes forskolin-induced SIRT1 phosphorylation. D, Phosphorylation of SIRT1 S434 induced by forskolin, epinephrine, or clenbuterol is blocked by the PKA inhibitor, H-89. E, Removal of forskolin attenuates cAMP signaling and SIRT1 phosphorylation and re-establishes PGC-1α acetylation. Transfected, FLAG-tagged PGC-1α was immunoprecipitated from U2OS cells treated with Fsk for 30 min, washed with PBS, and then incubated in Fsk-free medium for the indicated time points.