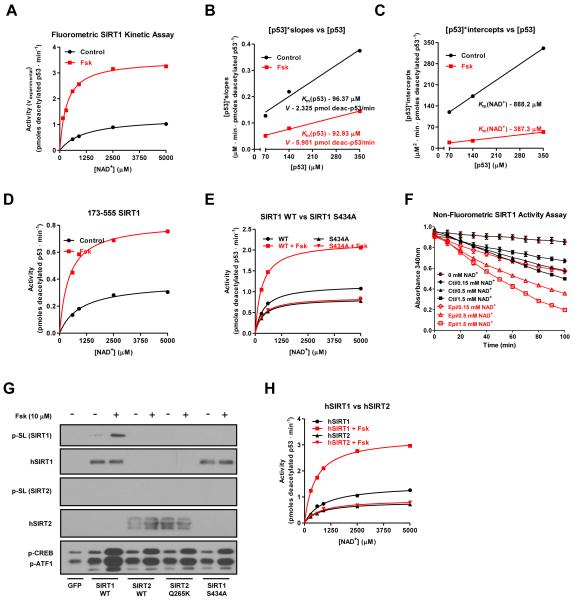
Figure 4. The cAMP/PKA pathway increases intrinsic SIRT1 enzymatic activity through serine 434 phosphorylation.
A, Forskolin treatment increases SIRT1 activity at multiple concentrations of acetylated substrate and three different NAD+ concentrations (140 μM acetyl-p53 peptide results are shown; data for 70 and 350 μM peptide are found in Figure S4B). Transfected FLAG-tagged SIRT1 was immunoprecipitated from DMSO or Fsk-treated U2OS cells and its activity was quantified using a fluorophore-conjugated acetylated p53 peptide; error bars are present but may be smaller than data point symbols. B-C, Forskolin treatment decreases the SIRT1 Km for NAD+ and increases the reaction rate, V, while leaving the Km for p53 unaffected. Kinetic data from Figure 4A were first plotted reciprocally as [NAD+]/vexperimental vs [NAD+] (Figure S4D) and the slopes and y-intercepts from that graph were used to make secondary plots from which the reaction velocity, V, and the Km’s for both p53 and NAD+ were calculated. V was converted from arbitrary fluorescence units to pmoles of deacetylated p53/min using a deacetylated standard (ENZO). D, The SIRT1 catalytic domain (amino acids 173-555) is sufficient to mediate the effects of forskolin on deacetylase activity. E, S434 mutation abolishes the effect of cAMP on SIRT1 in vitro activity. F, Activation of PKA signaling by epinephrine increases SIRT1 activity in a non-fluorometric based in vitro assay using a H3K14 peptide. G-H, Forskolin induces the phosphorylation and activation of human SIRT1 but not SIRT2 in vitro. Data are presented as means ± S.D.