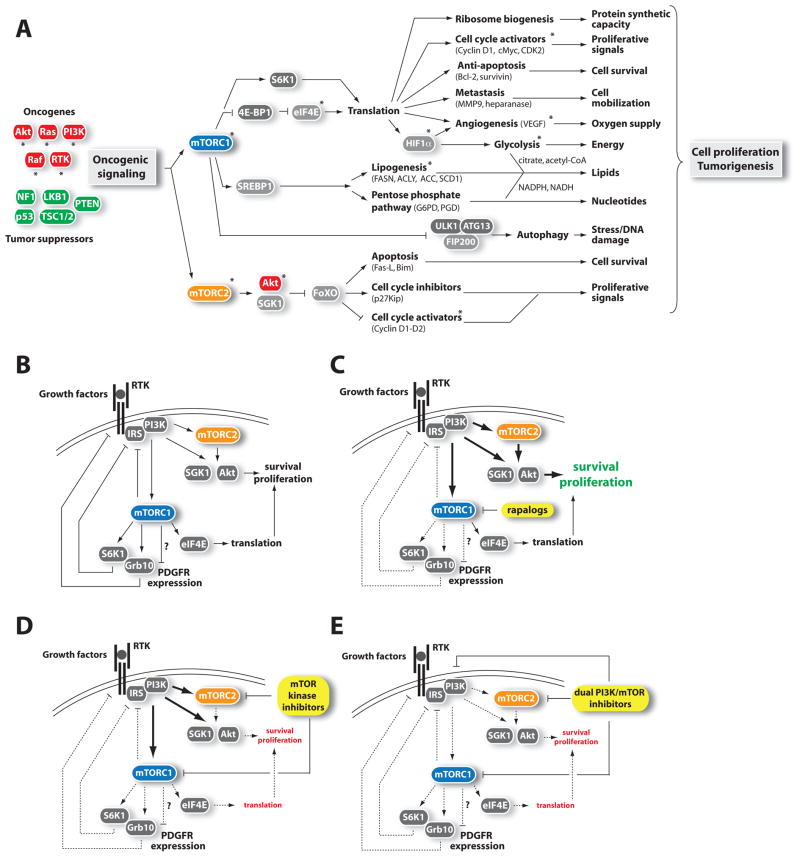
Figure 3. Connections of mTOR to cancer.
(A) mTOR signaling promotes tumorigenesis. Oncogenes (red) or tumor suppressors (green) implicated in the control of mTOR signaling are indicated. Asterisk (*) denotes proteins currently targeted for cancer therapy.
(B) mTORC1 controls many negative feedback loops that regulate RTK-PI3K signaling.
(C) The inhibition of mTORC1 by rapalogs reduces the intensity of the negative feedback loops on RTK signaling, which promotes PI3K activation and cell survival. Because the rapalogs only partially inhibit 4E-BP1 phosphorylation, their impact on eiF4E-mediated protein translation is limited.
(D) By completely blocking mTORC1, mTOR kinase inhibitors strongly inhibit the 4E-BP1/eIF4E axis and protein synthesis. Additionally, mTOR kinase inhibitors can affect cell survival and proliferation by blocking mTORC2-mediated Akt phosphorylation. The elevation in RTK-PI3K-PDK1 activity in response to mTOR kinase inhibitors can potentially reactivate Akt phosphorylation on Thr308, which may be sufficient to drive cell survival.
(E) Dual PI3K/mTOR inhibitors block all known outputs of the PI3K, mTORC1, and mTORC2 pathways.
Refer to the text and abbreviation list for details about the complete name of proteins.