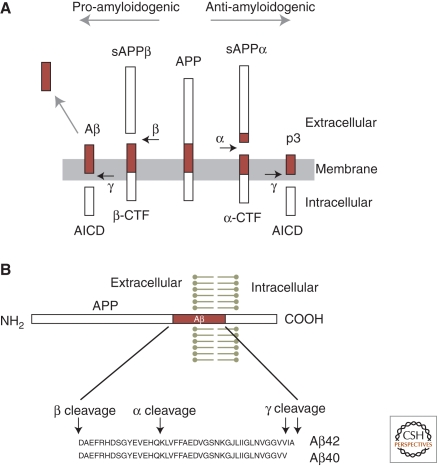
Figure 1.
APP processing and the formation of Aβ peptide. (A, middle) The full-length human amyloid precursor protein (APP), a single transmembrane protein with an intracellular carboxyl terminus. (Horizontal arrows) Specific protease cleavage sites. In the amyloidogenic pathway (to the left), sequential cleavage of APP by β-secretase and γ-secretase releases the soluble extracellular domain of APP (sAPPβ), Aβ peptide, and the intracellular carboxy-terminal domain of APP (AICD). Cleavage by α-secretase prevents formation of Aβ, instead producing sAPPα and p3 peptide. (CTF) Carboxy-terminal fragment of APP, before cleavage by γ-secretase. (B) Diagram of the APP polypeptide and sequence of Aβ40 and Aβ42 peptides, with secretase cleavage sites indicated.