Abstract
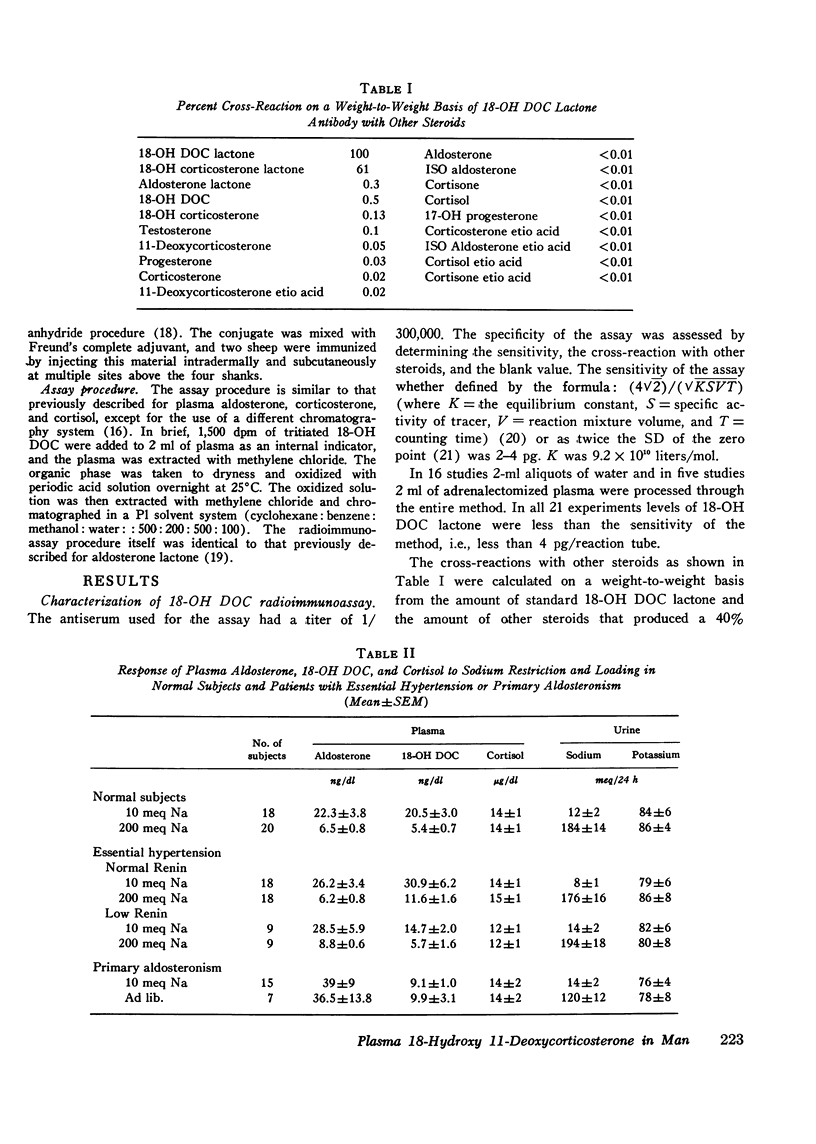
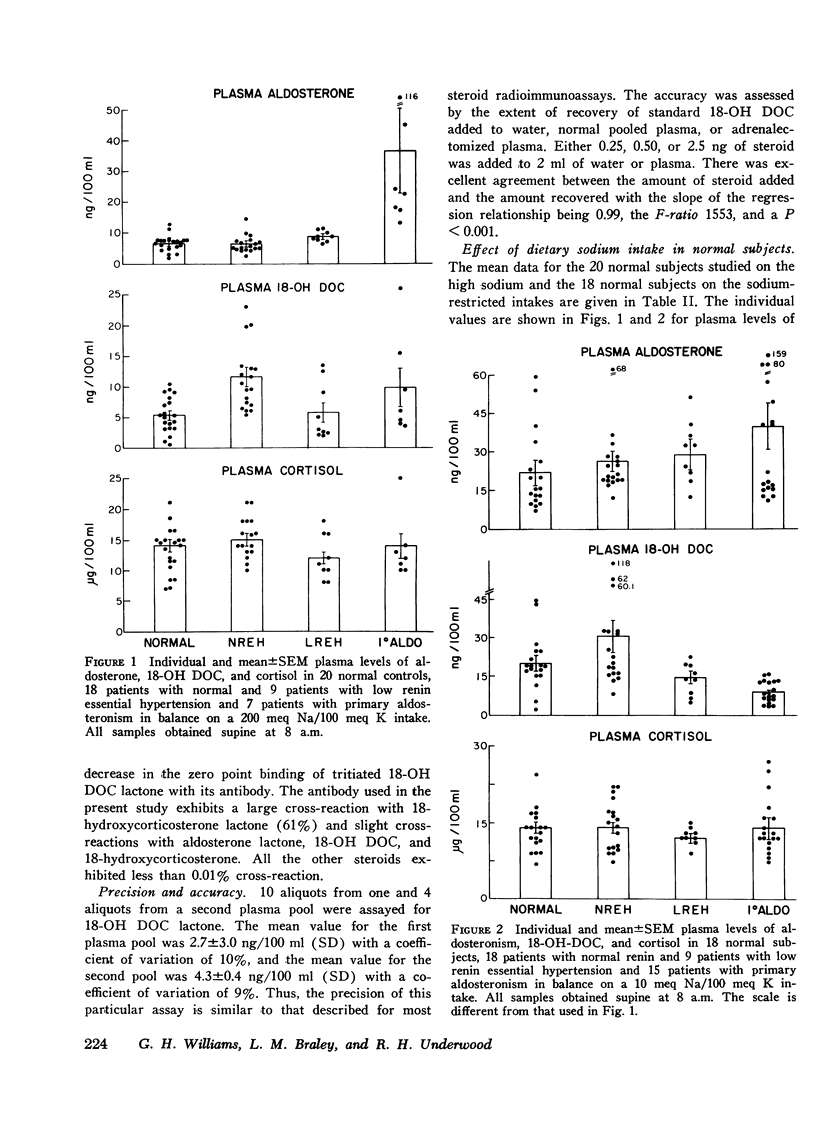
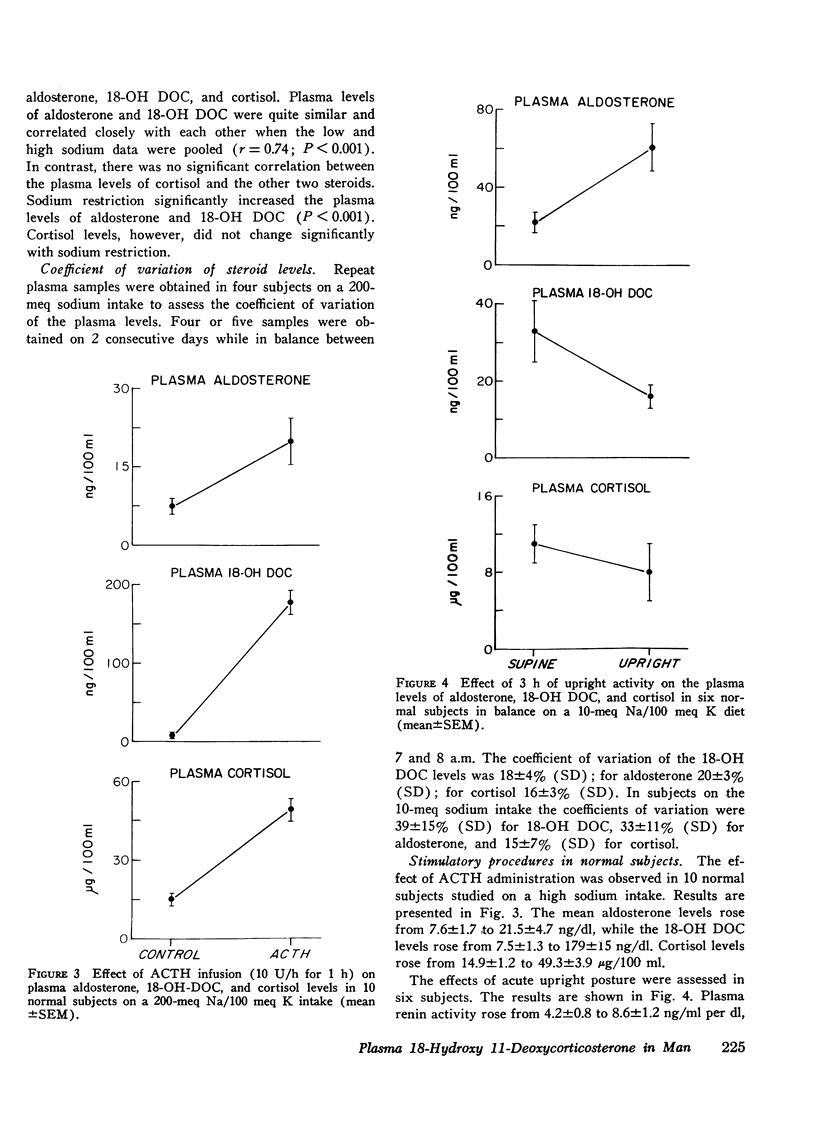
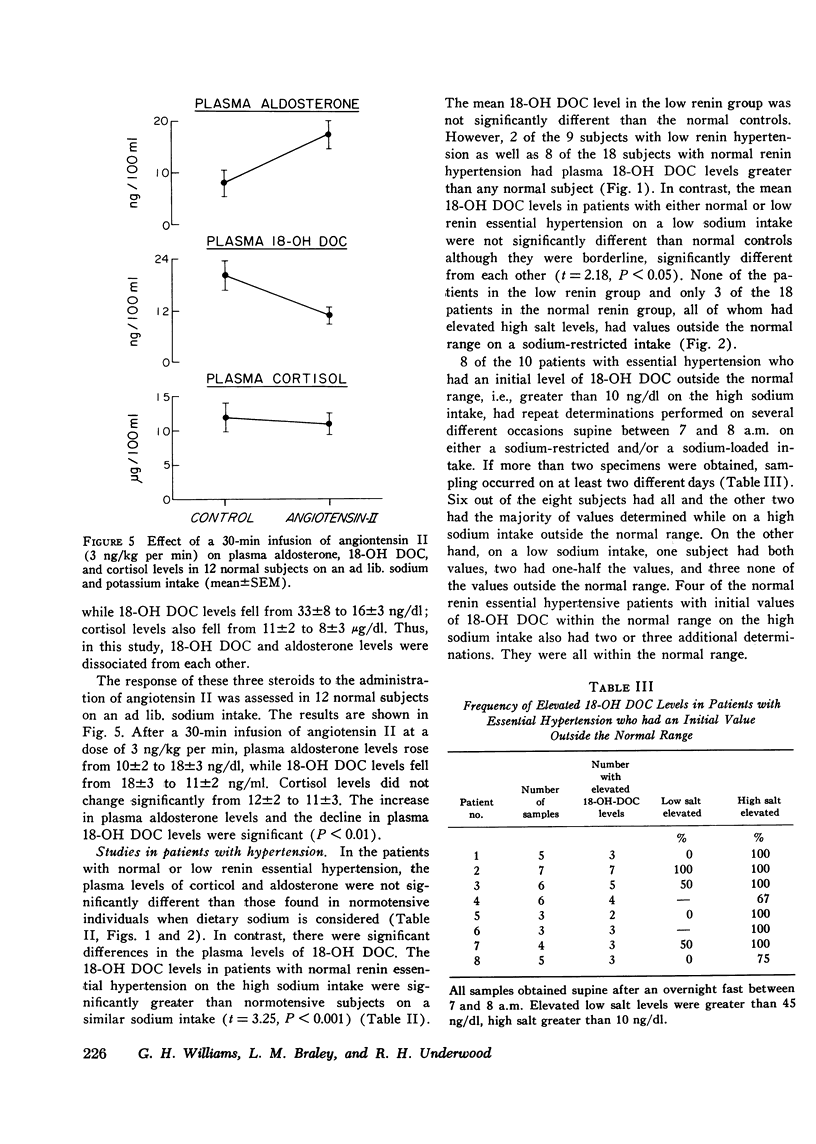
18-hydroxy 11-deoxycorticosterone (18-OH DOC), a weak mineralocorticoid, was estimated by a radioimmunoassay procedure after purification in 49 patients with hypertension and 38 normal control subjects. The sensitivity of the method was 2-4 pg; there was no detectable blank, and the precision was 9-10%. In normal subjects the absolute plasma levels were similar to those of aldosterone. ACTH administration produced a 23-fold increase, and sodium restriction resulted in a 4-fold increase (5.4+/-0.7-20.5+/-3.0 ng/dl). On the other hand, the plasma levels of 18-OH DOC declined by nearly 50% with upright posture or angiotensin II infusion. During both of these procedures, plasma aldosterone levels significantly increased. Patients with normal and low renin hypertension had similar changes in plasma 18-OH DOC levels with sodium restriction. However, the mean high sodium level in the normal renin essential hypertension group (11.6+/-1.6 ng/dl) was significantly greater (P is less than 0.001) than in the control group (5.4+/-0.7 ng/dl). In addition, at least 22% and perhaps as high as 37% of the hypertensive subjects had levels greater than the upper limits of normal on a high sodium intake. Differences between the groups were less impressive in the sodium-restricted studies. There were no significant differences in age, duration of hypertension, sodium balance, serum sodium, potassium, or blood urea nitrogen in those patients who had elevated levels of plasma 18-OH DOC. Patients with primary aldosteronism had levels within the normal range on both dietary intake. However, in contrast to the other groups there were no significant changes in the plasma levels with sodium restriction. Thus, a significant number of patients with essential hypertension presumably have an alteration in 18-OH DOC secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner H. R., Laragh J. H., Baer L., Newton M. A., Goodwin F. T., Krakoff L. R., Bard R. H., Bühler F. R. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972 Mar 2;286(9):441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- Crane M. G., Harris J. J., Johns V. J., Jr Hyporeninemic hypertension. Am J Med. 1972 Apr;52(4):457–466. doi: 10.1016/0002-9343(72)90036-8. [DOI] [PubMed] [Google Scholar]
- De Nicola A. F., Birmingham M. K. Biosynthesis of 18-hydroxydeoxycorticosterone from deoxycorticosterone-4-14C by the human adrenal gland. J Clin Endocrinol Metab. 1968 Sep;28(9):1380–1383. doi: 10.1210/jcem-28-9-1380. [DOI] [PubMed] [Google Scholar]
- De Nicola A. F., Oliver J. T., Birmingham M. K. Biotransformation of 1,2-3-H-11-deoxycorticosterone and 4-14-C-progesterone by rats with adrenal regeneration hypertension. Endocrinology. 1968 Jul;83(1):141–148. doi: 10.1210/endo-83-1-141. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., BOREK F., BEISER S. M., LIEBERMAN S. Steroid-protein conjugates. I. Preparation and characterization of conjugates of bovine serum albumin with testosterone and with cortisone. J Biol Chem. 1957 Oct;228(2):713–727. [PubMed] [Google Scholar]
- Emanuel R. L., Cain J. P., Williams G. H. Double antibody radioimmunoassay of renin activity and angiotensin II in human peripheral plasma. J Lab Clin Med. 1973 Apr;81(4):632–640. [PubMed] [Google Scholar]
- Fishman L. M., Küchel O., Liddle G. W., Michelakis A. M., Gordon R. D., Chick W. T. Incidence of primary aldosteronism uncomplicated "essential" hypertension. A prospective study with elevated aldosterone secretion and suppressed plasma renin activity used as diagnostic criteria. JAMA. 1968 Aug 12;205(7):497–502. doi: 10.1001/jama.205.7.497. [DOI] [PubMed] [Google Scholar]
- Jose A., Crout J. R., Kaplan N. M. Suppressed plasma renin activity in essential hypertension. Roles of plasma volume, blood pressure, and sympathetic nervous system. Ann Intern Med. 1970 Jan;72(1):9–16. doi: 10.7326/0003-4819-72-1-9. [DOI] [PubMed] [Google Scholar]
- Melby J. C., Dale S. L., Wilson T. E. 18-Hydroxy-deoxycorticosterone in human hypertension. Circ Res. 1971 May;28(5 Suppl):143–152. doi: 10.1161/01.res.28.5.ii-143. [DOI] [PubMed] [Google Scholar]
- Nowaczynski W., Kuchel O., Genest J., Messerli F. H., Honda M., Tolis G., Seth K., Parvin-Pande R., Kubo S., Grose J. Dynamic aldosterone and 18-hydroxydeoxycorticosterone studies in labile and stable benign essential hypertension. J Steroid Biochem. 1975 May;6(5):767–778. doi: 10.1016/0022-4731(75)90066-7. [DOI] [PubMed] [Google Scholar]
- PERON F. G. Isolation of 18-hydroxydeoxycorticosterone from rat adrenals. Endocrinology. 1961 Jul;69:39–45. doi: 10.1210/endo-69-1-39. [DOI] [PubMed] [Google Scholar]
- Rapp J. P., Dahl L. K. 18-Hydrox-deoxycorticosterone secretion in experimental hypertension in rats. Circ Res. 1971 May;28(5 Suppl):153–159. doi: 10.1161/01.res.28.5.ii-153. [DOI] [PubMed] [Google Scholar]
- Spark R. F., Melby J. C. Hypertension and low plasma renin activity: presumptive evidence for mineralocorticoid excess. Ann Intern Med. 1971 Dec;75(6):831–836. doi: 10.7326/0003-4819-75-6-831. [DOI] [PubMed] [Google Scholar]
- Tuck M. L., Dluhy R. G., Williams G. H. Sequential responses of the renin-angiotensin-aldosterone axis to acute postural change: effect of dietary sodium. J Lab Clin Med. 1975 Nov;86(5):754–763. [PubMed] [Google Scholar]
- Tuck M. L., Williams G. H., Cain J. P., Sullivan J. M., Dluhy R. G. Relation of age, diastolic pressure and known duration of hypertension to presence of low renin essential hypertension. Am J Cardiol. 1973 Oct;32(5):637–642. doi: 10.1016/s0002-9149(73)80056-6. [DOI] [PubMed] [Google Scholar]
- Underwood R. H., Williams G. H. The simultaneous measurement of aldosterone, cortisol, and corticosterone in human peripheral plasma by displacement analysis. J Lab Clin Med. 1972 May;79(5):848–862. [PubMed] [Google Scholar]
- Williams G. H., Cain J. P., Dluhy R. G., Underwood R. H. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest. 1972 Jul;51(7):1731–1742. doi: 10.1172/JCI106974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. H., McDonnell L. M., Tait S. A., Tait J. F. The effect of medium composition and in vitro stimuli on the conversion of corticosterone to aldosterone in rat glomerulosa tissue. Endocrinology. 1972 Oct;91(4):948–960. doi: 10.1210/endo-91-4-948. [DOI] [PubMed] [Google Scholar]
- Woods J. W., Liddle G. W., Michelakis A. M., Brill A. B. Effect of an adrenal inhibitor in hypertensive patients with suppressed renin. Arch Intern Med. 1969 Apr;123(4):366–370. [PubMed] [Google Scholar]


