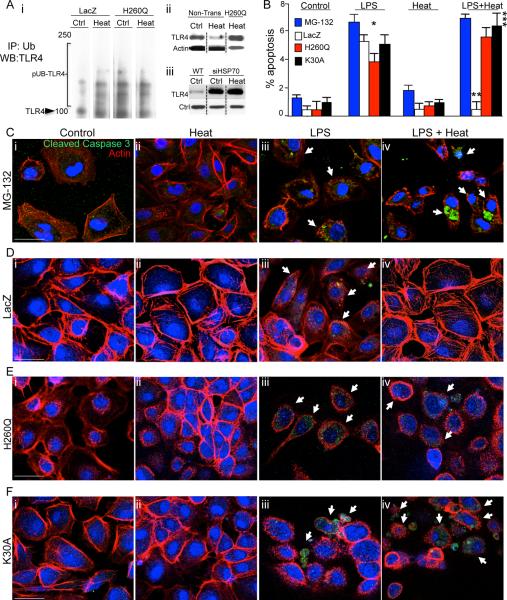
Figure 3. The induction of Hsp70 leads to the ubiquitination and degradation of TLR4 via the co-chaperone CHIP.
A i: Representative immunoblots in which LacZ and H260Q-transfected IEC-6 enterocytes were exposed to heat or were maintained at 37°C, then immunoprecipitated with anti-ubiquitin antibodies and immunoblotted with anti-TLR4 antibodies, displaying poly-ubiquitinated species (“pUB-TLR4”). The location of TLR4 on the gel is shown. ii: Representative SDS-PAGE of IEC-6 cells probed with anti-TLR4 antibodies that were either non-transfected or transfected with H260Q then maintained at 37°C or exposed to heat, in which heat exposure leads to a reduction in TLR4 expression that is not seen in H260Q-transfected cells. iii: SDS-PAGE showing expression of TLR4 and loading protein control in either wild-type (“WT”) IEC-6 cells or IEC-6 cells treated with siRNA to Hsp70 (“siHSP70”) that were either untreated (“Ctrl”) or treated with heat as in Methods (“Heat”). B–F: Representative confocal micrographs of IEC-6 enterocytes treated with MG-132 (C), or transduced with LacZ (D) or H260Q-CHIP (E) or K30A-CHIP (F) and treated as indicated and immuno-stained with cleaved-caspase 3 (green), β-actin (red) and DAPI (blue). Size bar = 10μ. Shown in (B) % apoptosis per HPF > 50 fields with over 50 cells/field.; *p<0.05 control (all bars) versus LPS (all bars); **p<0.05 LPS (open bar) versus LPS+Heat (open bar); vs LPS open bar, ***p<0.05 Control versus LPS + Heat (black, red and blue bars) in 3 separate experiments. Size bar=10μ. Arrows point to apoptotic cells in each group under the indicated treatment.