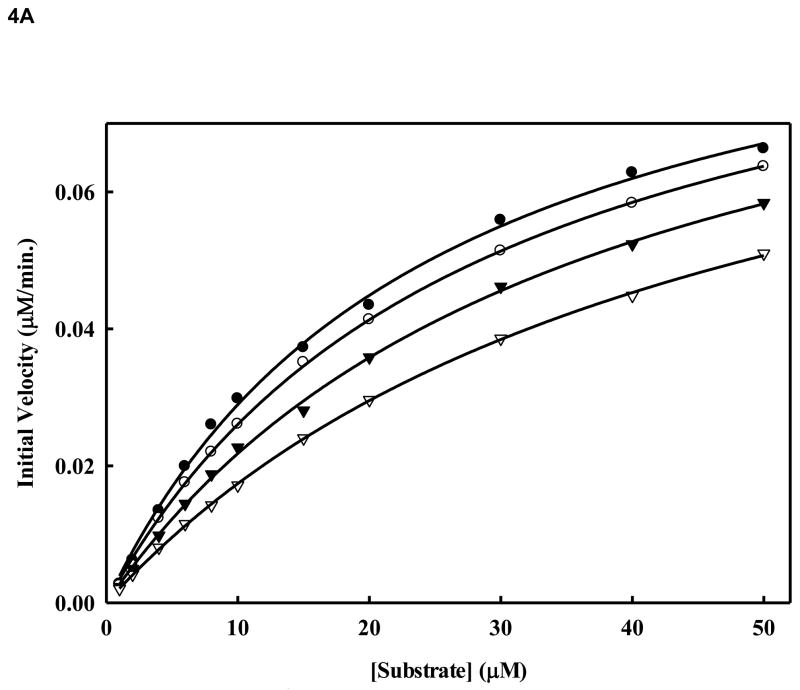
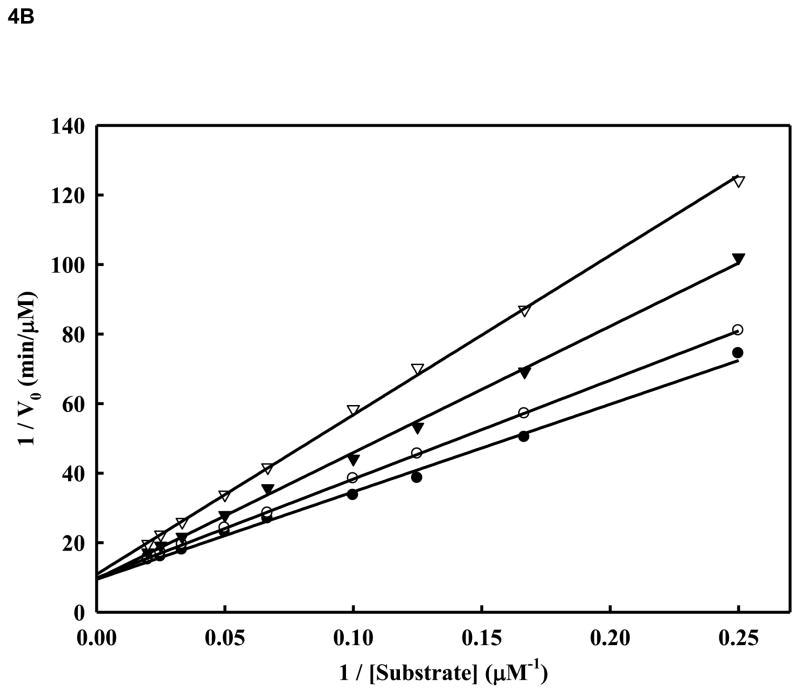
Figure 4. A. Inhibition of WNV NS2BH-NS3pro protease activity by compound 14.
Initial reaction rates of cleavage of the tetra-peptide-AMC substrate catalyzed by WNV NS2BH-NS3 protease (28 nM) in 200 mM Tris HCl (pH 9.5), 6.0 mM NaCl, 30 % glycerol and 0.1 % CHAPS at 37 C were determined by varying the tetra-peptide substrate concentrations in the range of 1, 2, 4, 6, 8, 10, 15, 20, 30, 40 and 50 μM at each concentration of inhibitor fixed at 0 (solid circle), 1.0 μM (open circle), 2.0 μM (solid triangle) and 5.0 μM (open triangle). The reactions were initiated by the addition of WNV NS2BH/NS3pro protease and the fluorescence intensity at 460 nm was monitored with an excitation at 380 nm. Reactions were less than 5% completion in all cases to maintain valid steady-state measurements. The solid lines are fitted lines using the Michaelis-Menten equation. B. Lineweaver-Burk plot of compound 14. The same experiments as described in Fig. 4A were used to plot this graph. The concentrations of the inhibitors are 0 (solid circle), 1.0 μM (open circle), 2.0 μM (solid triangle) and 5.0 μM (open triangle), respectively. The concentrations of tetra-peptide substrate are (4, 6, 8, 10, 15, 20, 30, 40 and 50 μM range).