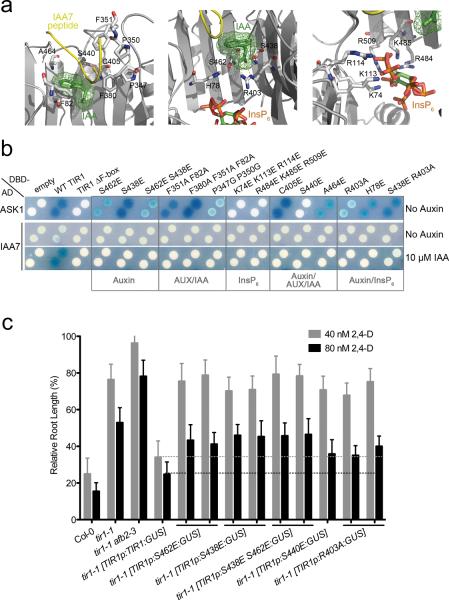
Figure 4. Mutations at Auxin, Aux/IAA and InsP6 Binding Sites Impair Auxin-Dependent TIR1-Aux/IAA Interaction And Compromise TIR1 Function In Vivo.
a. Side view of auxin (green) and IAA7 peptide (yellow) (left), auxin and InsP6 (rainbow) (middle), and InsP6 (right) binding sites in TIR1 (grey). Selected ligand-interacting residues in TIR1 are shown in mostly white stick representation. b. Yeast-two hybrid ASK1, and auxin-induced IAA7 interactions with wild type (WT) TIR1 or TIR1 carrying mutations on ligand-binding sites. c. Five-day-old seedlings grown on MS media were transferred to media containing either 40 or 80 nM 2,4-D. Root elongation was measured after additional 4 days and expressed as a proportion of growth in the absence of auxin (scale bars represent STDEV). Auxin inhibits root growth elongation in wild type plants (Col-0) but tir1-1, as well as the tir1-1afb2–3 auxin-receptor mutants are resistant to auxin treatment. Auxin resistance in tir1-1 mutants is reverted by introducing TIR1 wild type fused to the β-glucuronidase (GUS) reporter gene, under the TIR1 promoter. Interrupted lines indicate that unlike TIR1p:TIR1:GUS, versions of TIR1 that carry mutations in ligand binding sites are unable to restore auxin sensitivity in tir1-1.