SUMMARY
Increased translocation of intestinal bacteria is a hallmark of chronic liver disease and contributes to hepatic inflammation and fibrosis. Here we tested the hypothesis that the intestinal microbiota and Toll-like receptors (TLRs) promote hepatocellular carcinoma (HCC), a long-term consequence of chronic liver injury, inflammation and fibrosis. Hepatocarcinogenesis in chronically injured livers depended on the intestinal microbiota, and TLR4 activation in non-bone marrow-derived resident liver cells. TLR4 and the intestinal microbiota were not required for HCC initiation but for HCC promotion, mediating increased proliferation, expression of the hepatomitogen epiregulin, and prevention of apoptosis. Gut sterilization restricted to late stages of hepatocarcinogenesis reduced HCC suggesting that the intestinal microbiota and TLR4 represent therapeutic targets for HCC prevention in advanced liver disease.
INTRODUCTION
Increased translocation of intestinal bacteria is common in patients with chronic liver disease, and causes characteristic infectious complications in advanced disease stages (Cirera et al., 2001; Schuppan and Afdhal, 2008; Tandon and Garcia-Tsao, 2008). In addition, translocation of bacterial components termed pathogen-associated molecular patterns (PAMPs) triggers inflammatory responses through Toll-like receptors (TLRs) in both early and late disease stages (Dolganiuc et al., 2007; Fukui et al., 1991; Nolan and Leibowitz, 1978; Seki et al., 2007; Wiest and Garcia-Tsao, 2005). Moreover, PAMPs from the intestinal microbiota and TLR4 contribute to liver fibrosis and cirrhosis (Broitman et al., 1964; Rutenburg et al., 1957; Seki et al., 2007). Notably, 80% of HCCs develop in fibrotic or cirrhotic livers as a consequence of chronic liver injury (Fattovich et al., 2004; Luedde and Schwabe, 2011) with HCC being the leading cause of death in patients with compensated liver cirrhosis (Fattovich et al., 2004). However, pathways that are responsible for the high rate of HCC development in the chronically injured and fibrotic liver remain largely unknown. Here, we hypothesize that inflammatory and fibrogenic responses induced by the gut microbiota and TLRs contribute to hepatocarcinogenesis, and might represent targets for HCC prevention or treatment.
RESULTS
TLR4 is required for hepatocarcinogenesis in the chronically injured liver
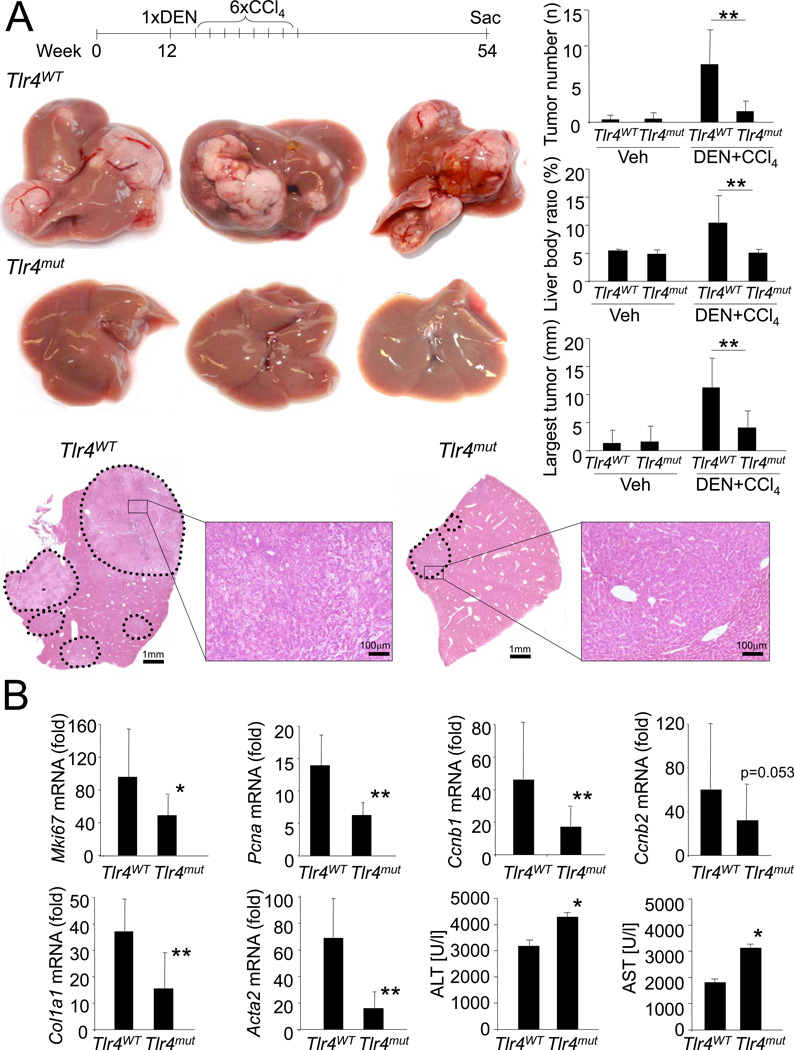
To determine whether the known contribution of TLRs to hepatic inflammation and fibrosis may set the stage for HCC development, we subjected C3H/HeJ and C3H/HeOuJ mice, which carry respectively a non-functional and the wild type Tlr4, to a combination of diethylnitrosamine (DEN) and the hepatotoxin carbon tetrachloride (CCl4). This model incorporates chronic injury, inflammation, fibrogenesis and CCl4-mediated increases of endotoxin levels (Seki et al., 2007), and thus shares several features with the microenvironment in which the majority of human HCCs arise. Tumors developing by this regimen showed typical features of HCC and microarray analysis revealed a characteristic HCC expression profile with Afp, Gpc3 and Cdkn2b among the most upregulated genes with greater than 90-fold increase in comparison to normal liver (data not shown). TLR4-wild-type (Tlr4WT) and TLR4-inactivated mutant (Tlr4mut) mice had a similar tumor incidence (100% in Tlr4WT mice, 78% in Tlr4mut mice, p=0.2105), but Tlr4mut mice displayed an 80–90% reduction of tumor number and size, and an almost normal liver-body weight ratio 10.5 months after the initial DEN injection (Figure 1A).
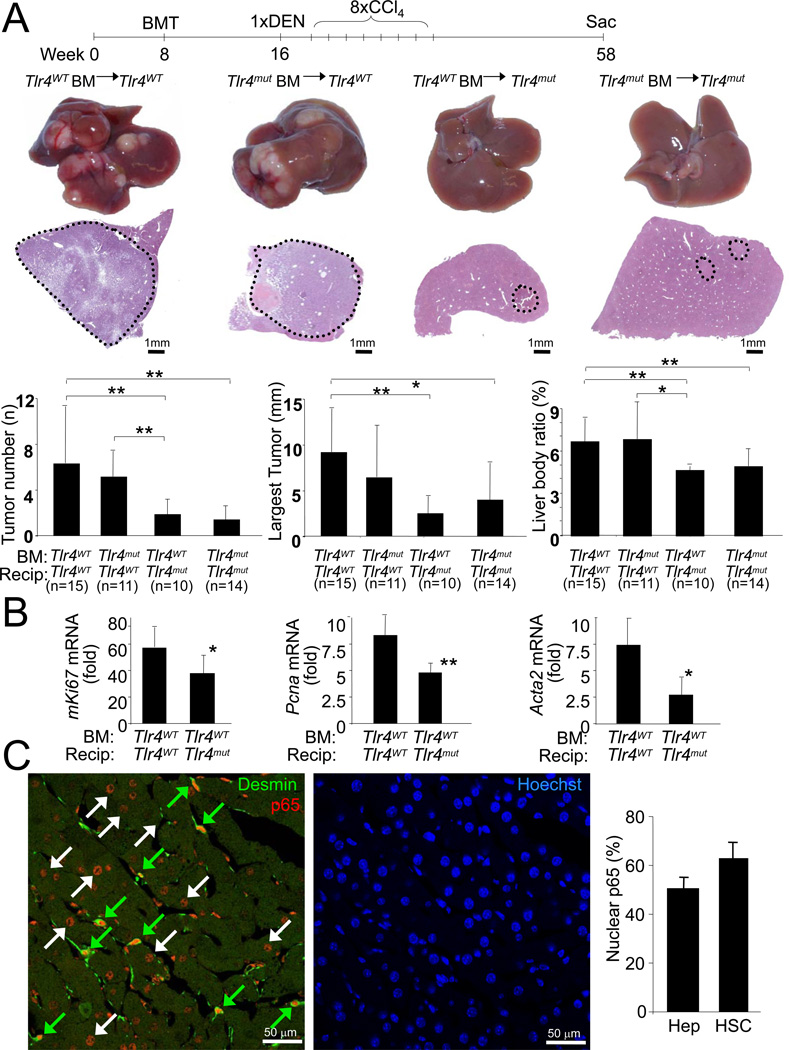
Figure 1. TLR4 contributes to hepatocarcinogenesis.
A. Tlr4WT mice (n=10) and Tlr4mut (n=9) were injected with DEN (100 mg/kg i.p.) at the age of 12 weeks followed by 6 injections of CCl4 (0.5 ml/kg i.p.) and sacrificed 10.5 months after DEN. Shown are tumor number, largest tumor size, liver-body weight ratio, H&E sections and representative images. B. Tlr4WT (n=14) and Tlr4mut mice (n=15) were treated with DEN followed by 2 injections of CCl4. Gene expression and ALT and AST levels were determined 48h after the second CCl4 injection. Fold induction in comparison to untreated livers. Data are represented as means ± SD. * p<0.05, ** p<0.01. See also Figure S1.
Taking advantage of the separate initiation and promotion stages in this model, we further analyzed the contribution of TLR4 at these stages. Following DEN injection, we observed no differences in the induction of inflammatory genes Il6 and Ccl2, and p53-dependent genes Cdkn1a and Bax between Tlr4WT and Tlr4mut mice (Figure S1A). DEN-induced liver injury, as determined by serum ALT and AST levels, was exacerbated in Tlr4mut mice (Figure S1A). However, the increased liver injury is unlikely to cause decreased hepatocarcinogenesis in Tlr4mut mice as liver injury after DEN is thought to promote hepatocarcinogenesis by inducing regenerative responses (Maeda et al., 2005; Naugler et al., 2007). Moreover, despite a small trend towards decreased HCC development, all Tlr4mut mice subjected to a purely genotoxic hepatocarcinogenesis protocol developed numerous large tumors (Fig.S1B). Thus, TLR4 status exerts no major effect on genotoxic tumor initiation apart from an increased susceptibility of Tlr4mut mice to liver injury. In contrast, we observed a significant impact of TLR4 status on tumor promotion by CCl4 with proliferation markers mKi67, Pcna, Ccnb1 and Ccnb2, hepatic fibrogenesis markers Col1a1 and Acta2 and inflammatory markers Il6, Il1b, Tnf and Ccl2 strongly reduced in Tlr4mut mice, as evidenced by microarray (data not shown) and qPCR analysis, despite increased liver injury (Figure 1B, Figure S1C–D). Similar differences were observed after DEN plus 4 CCl4 injections suggesting a lasting effect of absent TLR4 signaling (Figure S1D). While CCl4 plus DEN-induced liver injury was increased in Tlr4mut mice 48h after the last CCl4 injection, it was similar to Tlr4WT at earlier time points (Figure S1E) suggesting that the Tlr4mut mice have a defect in DEN plus CCl4-induced injury resolution and wound healing rather than a higher susceptibility to the initial injury. As chronic regeneration contributes to replication-induced mutagenesis and HCC, we confirmed the reduction of proliferation using Tlr4 knockout (Tlr4KO) mice in C57Bl/6 background and Ki-67 immunohistochemistry (Figure S1F–G).
To exclude that our findings reflect a particular role for TLR4 in the response to CCl4, we tested two additional models in which DEN was combined with thioacetamide or a choline-deficient diet, and found essentially the same results with decreased proliferation and fibrogenesis (Figure S1H–J). We also tested whether TLR2, which recognizes PAMPs from the gram-positive bacterial microbiota, contributes to hepatocarcinogenesis. However, we did not find a role for TLR2 in DEN plus CCl4-induced proliferation or HCC development (Figure S1K–L).
The intestinal microbiota and LPS promote hepatocarcinogenesis
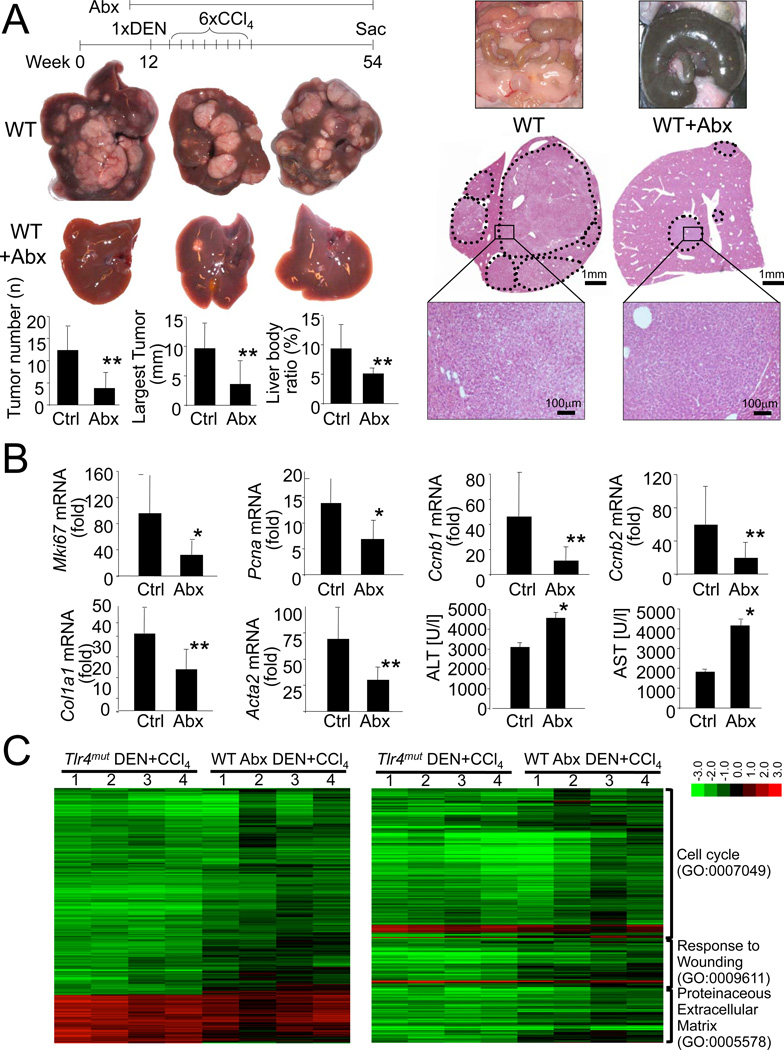
Next, we examined whether ligands from the bacterial intestinal microbiota are triggers for the observed TLR4-dependent tumor promotion. For this purpose, we gut-sterilized mice with a well-established cocktail of oral antibiotics that eliminates commensal bacteria (Rakoff-Nahoum et al., 2004) and reduces systemic LPS levels (Seki et al., 2007). This treatment not only resulted in >99.5% reduction of 16s levels in the cecum (Figure S2A) and an enlargement of the cecum commonly observed in germ-free mice (Figure 2A), but also a highly significant reduction of tumor number, size and the liver-body weight ratio in our DEN plus CCl4 HCC model (Figure 2A). Similar to data in the Tlr4mut mice, tumor incidence was not affected by gut sterilization (100% in gut-sterilized vs 100% in control mice). In addition to the similar degree of HCC reduction, gut-sterilization markedly pheno-copied events observed in the Tlr4mut mice such as reduced expression of cell cycle, fibrosis and inflammatory genes and increased liver injury (Figure 2B, Figure S2B). Accordingly, the great majority of 1752 genes, that were significantly up- or downregulated by DEN plus CCl4 and significantly up- or downregulated by TLR4 inactivation, were concordantly regulated in DEN plus CCl4-treated liver from gut-sterilized mice (r=0.794, p<0.0001) suggesting that TLR4 inactivation and gut sterilization exert highly similar biological effects on the liver during hepatocarcinogenesis (Figure 2C). Like Tlr4mut mice, gut-sterilized mice also did not show differences in inflammatory and p53-dependent gene expression in response to acute DEN treatment but displayed increased liver injury (Figure S2C).
Figure 2. The intestinal microbiota promotes hepatocarcinogenesis.
A. WT mice (C3H/HeOuJ) were treated with DEN plus 6 injections of CCl4 and either did (n=11) or did not (n=7) receive antibiotics (ampicillin, neomycin, metronidazole, vancomycin) in their drinking water starting 2 weeks before and throughout the entire period. Shown are representative images of ceca, tumors and H&E sections. B. WT mice were treated with DEN plus 2 injections of CCl4, and either did (“Abx”, n=14) or did not (“Ctrl”, n=9) receive the above-described antibiotics cocktail 2 weeks before DEN injection until they were sacrificed 48h after the last CCl4 injection. Gene expression is expressed as fold induction in comparison to untreated liver. Liver injury was assessed by ALT and AST measurements C. Tlr4WT, Tlr4mut and Tlr4WT mice treated with antibiotics were injected with DEN and two subsequent injections of CCl4 and sacrificed 48h later. RNA of livers from untreated Tlr4WT mice (n=3), DEN plus CCl4-treated Tlr4WT (n=4), DEN plus CCl4-treated Tlr4mut mice (n=4) and antibiotics plus DEN plus CCl4-treated Tlr4WT mice (n=4) were used for microarray analysis. The left heatmap shows changes of 1752 genes in the Tlr4mut and antibiotics-treated mice that were (i) at least 1.8-fold up- or downregulated by DEN plus CCl4 treatment in Tlr4WT mice in comparison to untreated mice, and (ii) 1.8-fold up- or downregulated in DEN plus CCl4-treated Tlr4mut mice in comparison to DEN plus CCl4-treated Tlr4WT mice. The right heatmap shows genes fulfilling the same conditions as described above restricted to the GO categories 0007049, 0009611 and 0005578. Data are represented as means ± SD. * p<0.05, ** p<0.01. See also Figure S2.
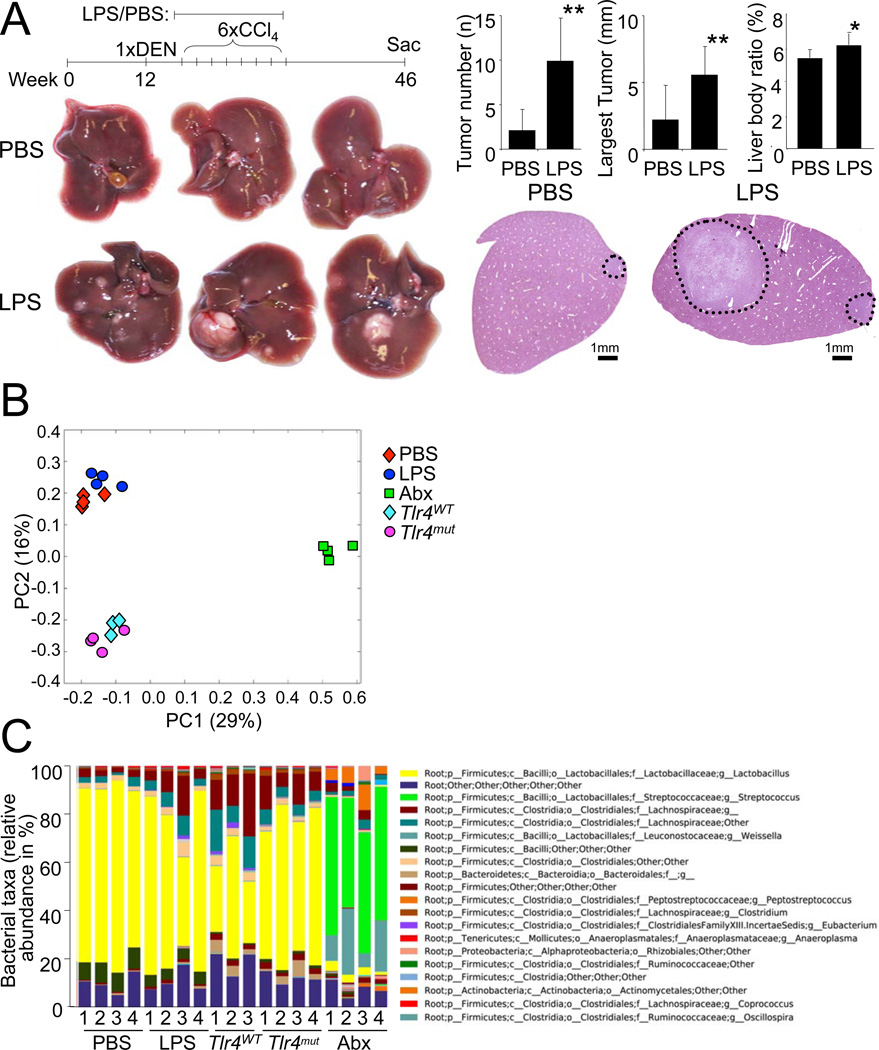
As a third approach in addition to TLR4 inactivation and gut sterilization, we treated mice with a low, non-toxic dose of LPS via subcutaneous osmotic pumps for 12 weeks during DEN plus CCl4-induced hepatocarcinogenesis. This low-dose LPS treatment led to a significant increase in inflammatory gene expression, tumor number, tumor size and liver-body weight ratio (Figure 3A, Figure S3) further confirming our hypothesis that the LPS-TLR4 pathway promotes hepatocarcinogenesis. As Tlr5-deficient mice have an altered gut microbiome (Vijay-Kumar et al., 2010), it is conceivable that TLR4 might also affect the composition of the gut microbiome and thereby influence HCC development rather than exerting direct effects in the liver. To address this possibility, we first performed 16s-based pyrosequencing of the cecal gut microbiota of wild-type and Tlr4mut mice as well as mice treated with PBS or LPS. Unifrac analysis revealed no significant differences between Tlr4WT and Tlr4mut mice, and between PBS- and LPS-treated mice, but showed distinct clustering of the gut-sterilized mice (Figure 3B). Interestingly, differences between these 2 independent experiments were larger in the Unifrac analysis than differences between Tlr4WT and Tlr4mut mice, or PBS- and LPS-treated mice again underlining that LPS and TLR4 status exert no significant influence on the composition of the gut microbiome. Moreover, the taxonomic distribution of the cecal gut microbiome was similar between Tlr4WT and Tlr4mut mice, and PBS- and LPS-treated mice (Figure 3C) and among the 118 detected taxa at the genus level, we found no significant differences between Tlr4WT and Tlr4mut mice, and only one taxon (firmicutes;bacilli;other;other;other) with a significant but modest difference between PBS-treated mice (8.1% abundance) and LPS-treated mice (4.7% abundance). However, as there was no difference in this taxon between Tlr4WT and Tlr4mut mice, it is extremely unlikely that the different abundance of this taxon is responsible for differences in hepatocarcinogenesis.
Figure 3. LPS promotes hepatocarcinogenesis but does not change the intestinal bacterial microbiota.
A. Tlr4WT (C3H/HeOuJ) mice were treated with DEN plus 6 injections of CCl4 and either received LPS (300 µg/kg/d, n=9) or PBS (n=10) via subcutaneous pumps starting one week before the first CCl4 injection for 12 weeks. Mice were sacrificed 8.5 months after DEN injection to determine tumor number, size and liver-body weight ratio. * p<0.05, ** p<0.01. B. Unifrac analysis of 16s sequencing results of the cecal microbiome in Tlr4WT (n=3) and Tlr4mut (n=4) mice, Tlr4WT mice treated with subcutaneous PBS (n=4) or LPS (n=4) pumps for 2 weeks, or Tlr4WT mice receiving antibiotics (n=4) for 2 weeks. C. Taxonomic distribution of the cecal microbiome in Tlr4WT and Tlr4mut mice, Tlr4WT mice treated with subcutaneous PBS or LPS pumps for 2 weeks, or mice receiving antibiotics for 2 weeks. The chart contains a total of 118 taxa at the genus level and the top 20 taxa are annotated on the left with most abundant taxa listed on top. Data are represented as means ± SD. * p<0.05, ** p<0.01. See also Figure S3.
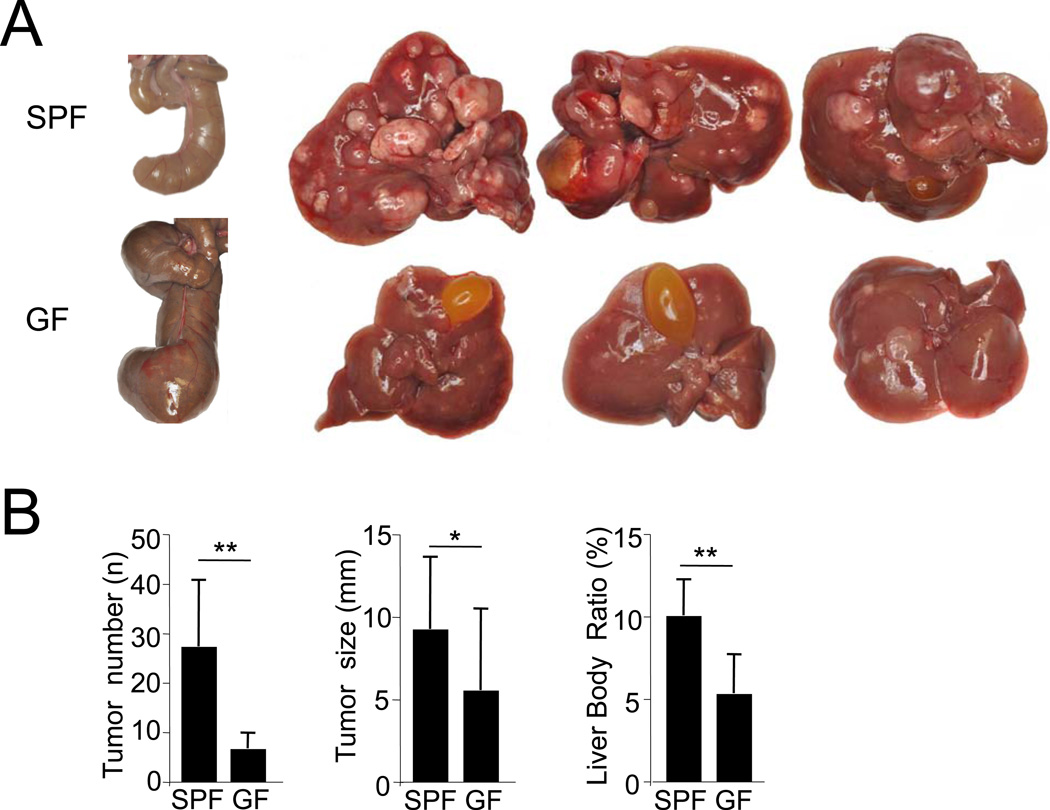
As a fourth approach, we investigated DEN plus CCl4-induced hepatocarcinogenesis in germ-free (GF) and littermate controls that had been transferred to a specific-pathogen free (SPF) environment after the initial DEN injection. GF mice displayed the typical enlargement of the cecum, and had a significantly reduced tumor number, tumor size and liver-body weight ratio in comparison to SPF mice (Figure 4A–B) thus confirming results in mice gut-sterilized by antibiotics, and excluding that effects of antibiotics on the liver were responsible for HCC reduction.
Figure 4. Germ-free status decreases hepatocarcinogenesis.
Germ-free (GF) C57Bl/6 mice were subjected to DEN (25 mg/kg i.p.) injection, split into specific-pathogen free (SPF) and GF groups, and then received 10 CCl4 (0.5 ml/kg i.p.) injections. A. Ceca of GF and SPF mice are shown on the far left and representative livers with tumors are shown on the right. B. Tumor number, size and liver-body weight ratio was determined in SPF mice (n=9) and GF mice (n=10). Data are represented as means ± SD. * p<0.05, ** p<0.01.
Non-bone marrow-derived resident hepatic cells mediate the tumor-promoting effect of TLR4
To determine the hepatic cell that promotes HCC in response to TLR4 activation, we generated TLR4-chimeric mice using a combination of irradiation, bone marrow transplantation (BMT) and liposomal clodronate-mediated macrophage depletion (Seki et al., 2009; Seki et al., 2007). This protocol achieved >97% suppression of LPS responses after transplantation of Tlr4mut bone marrow (BM) into Tlr4WT mice, almost complete restoration of LPS responses after transplantation of Tlr4WT BM into Tlr4mut mice and substitution of F4/80-positive hepatic macrophages (Figure S4A). DEN plus CCl4-induced hepatocarcinogenesis in TLR4-chimeric mice revealed no significant reduction of HCC number, size and liver-body weight ratio in mice transplanted with Tlr4mut BM, whereas TLR4 inactivation in resident (defined hereby as non-bone marrow-derived) liver cells significantly reduced HCC number, size and liver-body weight ratio (Figure 5A). Similarly, the induction of mKi67 and Pcna mRNA in response to acute DEN plus CCl4 treatment was significantly reduced in Tlr4mut mice with Tlr4WT BM in comparison to Tlr4WT mice with Tlr4WT BM (Figure 5B). Moreover, the depletion of hepatic macrophages by liposomal clodronate did not affect the response to DEN plus CCl4 as evident by mKi67 and Pcna mRNA levels (Figure S4B). To determine the resident liver cell that was responsive to LPS, a sublethal dose of LPS was injected into healthy mice and mice that had undergone DEN plus 8 CCl4 treatments, followed by determination of NF-κB p65 translocation. To avoid the known rapid release of TNF and subsequent TNF-mediated NF-κB activation as a cofounding factor, we employed either (i) TLR4-chimeric mice with Tlr4mut BM, (ii) macrophage-depleted mice or (iii) mice deficient for both TNFR1 and IL-1R1 for this experiment. In uninjured liver, we observed strong NF-κB p65 nuclear translocation in desmin-positive hepatic stellate cells and Kupffer cells but no translocation in hepatocytes after LPS injection (Figure S4C). In mice that had undergone DEN plus CCl4 treatment, we confirmed p65 nuclear translocation in hepatic stellate cells but also observed p65 translocation in a large percentage of hepatocytes (Figure 5C, Figure S4D) suggesting that both hepatocytes and hepatic stellate are candidates for TLR4-dependent tumor promotion in the chronically injured liver.
Figure 5. Resident liver cells mediate TLR4-dependent tumor promotion.
A. Tlr4WT and Tlr4mut mice were subjected to BMT (n≥10 per group) at 8 weeks followed by treatment with DEN plus 8 injections of CCl4. Mice were sacrificed 10.5 months after DEN. Shown are representative images of tumors and H&E sections, as well as tumor number, size and liver body weight ratio. B. Tlr4WT (n=7) and Tlr4mut mice (n=6) were transplanted with wild-type bone marrow and treated with DEN plus 2 injections of CCl4 at the age of 8 weeks. Proliferation markers were evaluated by qPCR and shown as fold induction in comparison to untreated mice. C. TLR4 chimeric mice with Tlr4WT resident cells and Tlr4mut BM (n=3) were treated with DEN plus CCl4 as described above. 10.5 months after DEN, LPS (10 mg/kg i.v.) was injected and NF-κB p65 nuclear translocation was determined by immunohistochemistry and confocal microscopy in non-tumor liver areas. Hepatic stellate cells were visualized by desmin staining and nuclei were visualized by Hoechst staining. Green and white arrows indicate NF-κB translocation in hepatic stellate cells and hepatocytes respectively. The percentage of hepatic stellate cells (HSC) and hepatocytes (Hep) with positive nuclear p65 was determined. Data are represented as means ± SD. * p<0.05, ** p<0.01. See also Figure S4.
Epiregulin is a TLR4-regulated hepatomitogen that promotes hepatocarcinogenesis
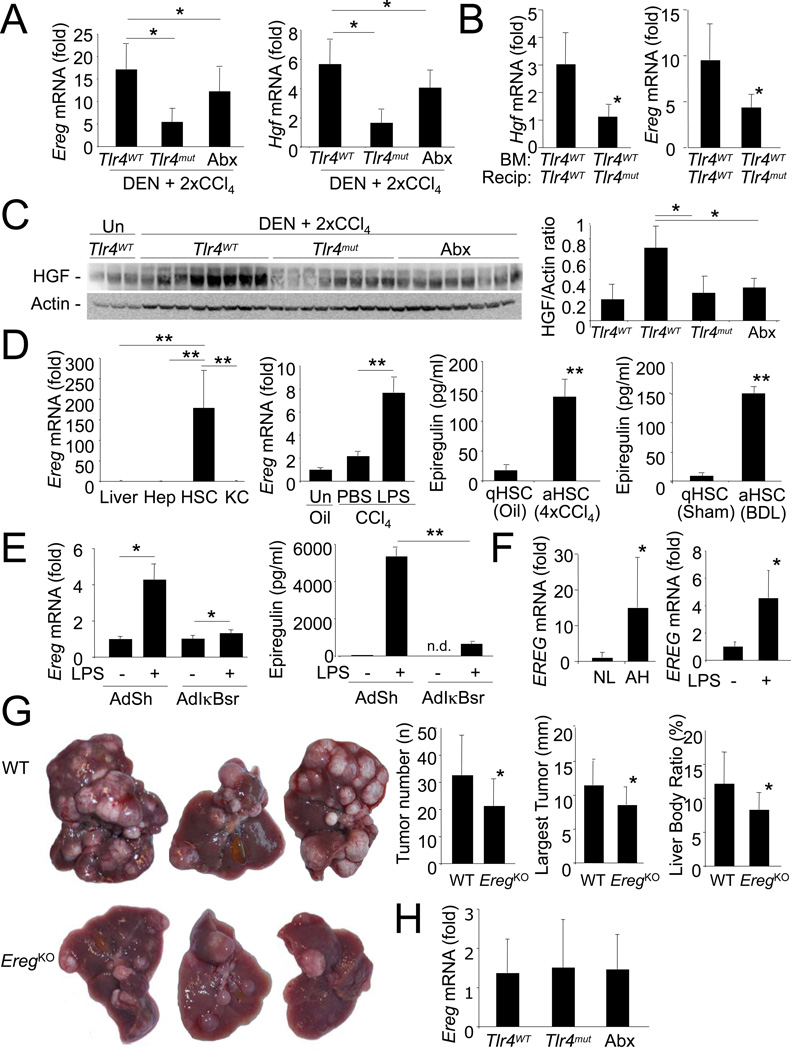
Microarray analysis demonstrated cell cycle-related genes as most significantly affected by TLR4 status or gut sterilization (Figure 2C). Our microarray and subsequent qPCR analysis revealed reduced expression of two hepatomitogens, HGF and epiregulin (Toyoda et al., 1995), in livers from DEN plus CCl4-treated Tlr4mut and gut-sterilized mice as well as from TLR4-chimeric mice with Tlr4mut in resident liver and Tlr4WT BM (Figure 6A–C). Both HGF mRNA (data not shown) and epiregulin mRNA were expressed much higher in hepatic stellate cells than in whole liver, hepatocytes or hepatic macrophages (Figure 6D). As the role of HGF in hepatocarcinogenesis remains controversial (Sakata et al., 1996; Santoni-Rugiu et al., 1996; Takami et al., 2007), we focused our attention on epiregulin. Hepatic stellate cells isolated from fibrotic livers secreted significantly more epiregulin than quiescent hepatic stellate cells (Figure 6D), and stimulation of hepatic stellate cells with LPS significantly upregulated epiregulin mRNA and protein in an NF-κB dependent manner (Figure 6E). Moreover, LPS injection into mice either as a bolus or via the above described chronic subcutaneous infusion also significantly increased epiregulin expression in the liver (Figure 6D, Figure S3). Epiregulin mRNA expression was also induced by LPS in human hepatic stellate cells and upregulated in livers of patients with alcoholic hepatitis, a group of patients with typically very high LPS levels (Fukui et al., 1991) (Figure 6F).
Figure 6. Epiregulin is an LPS-inducible promoter of hepatocarcinogenesis.
A. Ereg and Hgf mRNA were determined in Tlr4mut mice (n=14), Tlr4mut (n=15) and antibiotics-treated Tlr4WT (n=9) after DEN and 2×CCl4 injections. Fold induction vs. untreated livers. B. Ereg and Hgf mRNA were determined in TLR4-chimeric mice with Tlr4WT resident cells and Tlr4WT BM (n=6) or Tlr4mut resident cells and Tlr4WT BM (n=6) after treatment with DEN and 2 CCl4 injections. Fold induction vs. untreated livers. C. Western blot of HGF from Tlr4WT mice, Tlr4mut mice and antibiotics-treated mice after DEN and 2×CCl4 injections. D. Hepatocytes (Hep), Kupffer cells (KC) and hepatic stellate cells (HSC) were isolated from liver treated with DEN and 2 injections of CCl4 followed by analysis of Ereg mRNA expression by qPCR (left panel). Shown is a fold-induction vs. whole liver. Ereg mRNA was isolated in liver extracts from CCl4-treated mice injected with LPS (10 mg/kg i.v.) or PBS 4 days after the last CCl4 injection (second panel from left). Activated hepatic stellate cells were isolated from CCl4-treated and bile duct-ligated livers. Epiregulin was determined by ELISA 24hr after plating. E. Hepatic stellate cells from DEN plus 2×CCl4 treated mice were stimulated with LPS (100 ng/ml) in the presence of either an adenoviral IκB superrepressor (AdIκBsr) or empty shuttle virus (AdSh) by qPCR (left panel) or ELISA (right panel) F. EREG expression was determined in normal liver (“NL”, n=4) and livers of patients with alcoholic hepatitis (“AH”, n=10, left panel) and in activated human hepatic stellate cells stimulated with LPS (100 ng/ml, right panel). G. WT (n=11) and EregKO mice (n=14) were treated with DEN plus 22 injections of CCl4. Mice were sacrificed 6.5 months after DEN. Shown are representative images of tumors as well as tumor number, size of the largest tumor, and liver body weight ratio. H. Ereg expression was analyzed in non-tumorous regions of DEN plus CCl4-treated Tlr4WT (n=12), Tlr4mut mice (n=9) and antibiotics-treated Tlr4WT mice (n=6) 10.5 months after DEN. Fold induction vs. untreated liver. Data are represented as means ± SD. * p<0.05, ** p<0.01. nd, non-detectable
To elucidate the contribution of epiregulin to hepatocarcinogenesis, we subjected wild-type and epiregulin-deficient (EregKO) mice to DEN plus CCl4-mediated hepatocarcinogenesis. Due to the much lower propensity of C57Bl/6 mice to develop HCC, we used an altered protocol in which mice were subjected to DEN at day 15 postpartum followed by multiple CCl4 injections. EregKO mice displayed a significant reduction of tumor number, size and the liver-body weight ratio, albeit to a much lesser degree than Tlr4mut mice (Figure 6G). Moreover, epiregulin was not upregulated at late stages in our hepatocarcinogenesis model and did not differ between wild-type, Tlr4mut and gut-sterilized mice at these stages of hepatocarcinogenesis (Figure 6H). Thus, additional factors must be responsible for the striking reduction of hepatocarcinogenesis in Tlr4mut and gut-sterilized mice.
The intestinal microbiota and TLR4 suppress apoptosis in late stage of hepatocarcinogenesis
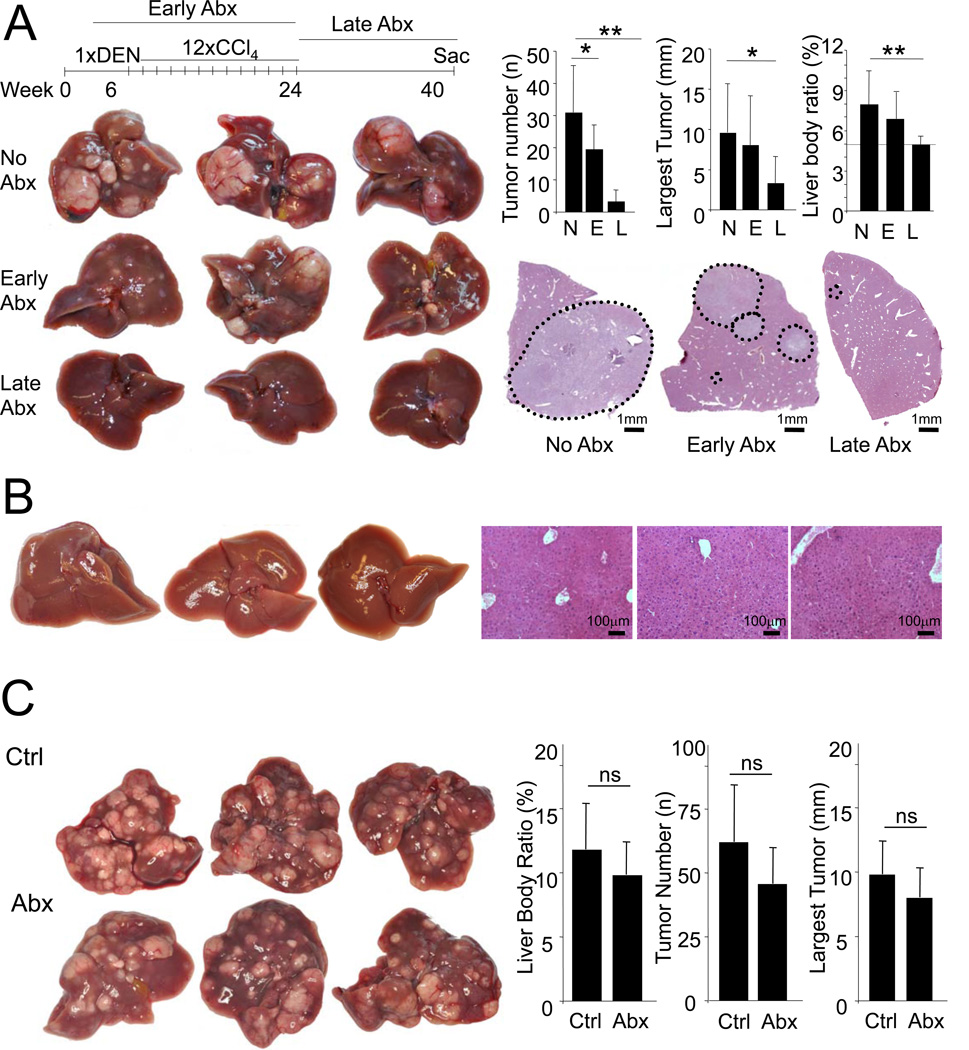
Next we attempted to establish the time frame during which bacterial PAMPs exert the most profound effects on hepatocarcinogenesis. Surprisingly, gut sterilization during the first 4.5 months of hepatocarcinogenesis showed only a moderate decrease of hepatocarcinogenesis (Figure 7A). In contrast, gut sterilization during the last 4 months of hepatocarcinogenesis reduced tumor number and size by 90% and 70%, respectively, and almost normalized the liver-body weight ratio (Figure 7A). At this point, mice had not yet developed macroscopically or microscopically visible tumors (Figure 7B) suggesting that gut sterilization prevented tumor development rather than leading to the regression of already established tumors. We additionally tested whether more selective gut decontamination with rifaximin, a widely used non-absorbable antibiotic for the prevention of hepatic encephalopathy in patients with end-stage liver disease (Bass et al., 2010), could influence hepatocarcinogenesis at late stages. We observed a borderline significant reduction of tumor numbers but saw no significant reduction in tumor size (data not shown) suggesting only moderate efficacy of rifaximin monotherapy on hepatocarcinogenesis at late stages (Figure S5).
Figure 7. The intestinal microbiota promotes HCC in late stages of hepatocarcinogenesis.
A. WT mice (C3H/HeOuJ) were treated with DEN (100 mg/kg i.p. at the age of 6 weeks) and 12 CCl4 (0.5 ml/kg i.p.) injections and were either not gut-sterilized (“N”, n=7), gut-sterilized starting 2 weeks before DEN injection until 2 weeks after the last CCl4 injection (“E”, n=10), or 2 weeks after the last CCl4 injection until they were sacrificed (“L”, n=8) followed by determination of tumor number, size and liver-body weight ratio. B. WT mice treated as described above were sacrificed at the time point when the gut-sterilization treatment was started to determine whether macroscopic or microscopic tumors were present. C. WT mice were treated with DEN (25 mg/kg i.p.) at day 15 postpartum followed by 14 weekly injections of CCl4. After confirming the presence of tumor by ultrasound (data not shown), mice were treated with (“Abx”, n=7) or without (“Ctrl”, n=6) antibiotics (ampicillin, vancomycin, neomycin and metronidazole) starting 2 weeks after the last CCl4 injection. After 6 weeks of antibiotics, mice were sacrificed and tumor number, size and liver-body weight ratio was determined. Data are represented as means ± SD. * p<0.05, ** p<0.01. ns, non-significant. See also Figure S5.
To determine whether gut-sterilization might affect already established tumors, we treated WT mice at a time point where tumors were already present with the quadruple antibiotics cocktail for 6 weeks. However, we found no significant change of tumor size, number or liver-body weight ratio in comparison to control mice (Figure 7C). When following single tumors by weekly ultrasound monitoring, we also saw no change in tumor size in antibiotics-treated mice (data not shown).
To further determine the mechanism by which the intestinal microbiota and TLR4 promote hepatocarcinogenesis, we analyzed gene expression, proliferation and apoptosis in gut-sterilized and Tlr4mut mice. Due to the lower tumor number and size, the overall amount of Ki-67 staining in Tlr4mut and gut-sterilized mice was expectedly lower (data not shown). Microarray comparison showed a highly similar gene expression pattern in HCC from wild-type, Tlr4mut and gut-sterilized mice (Figure S6A), and HCCs from these groups did not cluster separately as shown by dendrogram and Unifrac analysis (Figure S6B–C). There was a small but non-significant trend towards lower AFP and Ki-67 in HCCs from gut-sterilized mice and Tlr4mut mice which was most likely due to the smaller size of the tumors in these groups. There were no genes that showed significant changes, as defined by a corrected p-value of p<0.05, in the Tlr4mut or gut-sterilized HCC group when compared to the Tlr4WT HCC group (data not shown). Moreover, there were no differences in tumor grade (Figure S6D), and there was a similar loss of collagen IV staining in HCCs from all groups (Figure S6E).
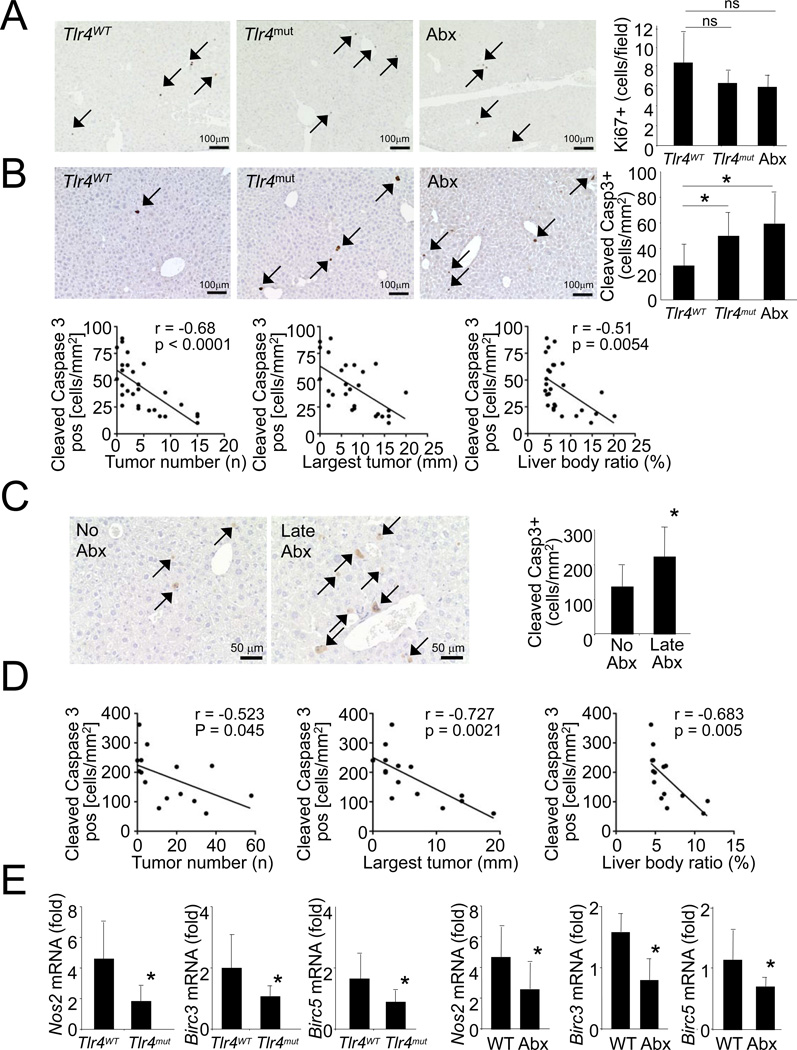
Microarray analysis showed no differences in expression of the cancer stem cell marker prominin 1/CD133 (data not shown), and we found only small and very rare clusters of cells positive for the progenitor marker A6 (Engelhardt et al., 1990) with mostly low A6 expression and morphology of intermediate hepatobiliary cells (Figure S6F). Similarly, there were only few cells with strong pan-cytokeratin signal typical for oval cells in tumors of all treatment groups and a larger but highly variable amount of cells with weak pan-cytokeratin expression typical of intermediate hepatobiliary cells in all groups (Figure S6G-H). Altogether, the microarray and immunohistochemical data suggest that TLR4 signaling is unlikely to promote hepatocarcinogenesis via progenitor cells. There was also no significant difference in Ki-67 positive cells in non-tumor liver between these groups suggesting that TLR4 and the intestinal microbiota do not promote tumor formation by increasing proliferation in non-tumor liver (Figure 8A).
Figure 8. TLR4 and the intestinal microbiota protect from apoptosis.
A–B. Livers from Tlr4WT (n=11), Tlr4mut (n=9) or antibiotics-treated Tlr4mut mice (n=7) were stained for Ki-67 (A.) or cleaved-caspase 3 (B.) followed by quantification. Correlation between the number of cleaved-caspase 3 positive cells and tumor number, size and liver-body weight ratio was determined. C–D. Tlr4WT were either not gut-sterilized (n=7), or gut-sterilized 2 weeks after the last CCl4 injection until they were sacrificed (n=8). Livers were stained for Ki-67 and cleaved-caspase 3 followed by quantification (B). Correlation between the number of cleaved-caspase 3 positive cells and tumor number, size and liver-body weight ratio was determined (C). E. Nos2, Birc3 and Birc5 mRNA levels were compared between livers from Tlr4WT (n=12) and Tlr4mut (n=9), and between untreated (n=9) and antibiotics treated (n=6) Tlr4WT. Data are represented as means ± SD. * p<0.05. See also Figure S6.
Both gut-sterilized and Tlr4mut mice displayed a significant increase in cleaved-caspase 3-positive cells with predominant expression of cleaved-caspase 3 in hepatocytes in non-tumor areas (Figure 8B). Notably, the number of cleaved caspase 3-positive cells was inversely and highly significantly correlated with tumor number, size and the liver-body weight ratio (Figure 8B) further underlining the biological relevance of these findings. Significant increases in cleaved-caspase 3 positive cells and a strong and highly significant inverse correlation between cleaved-caspase 3 and tumor number, size and liver-body weight ratio were also observed in mice that were gut-sterilized at late time points (Figure 8CD). Moreover, the expression of NF-κB-regulated anti-apoptotic genes Birc3, Birc5 and Nos2 (Hatano et al., 2001; Wang et al., 1998) was reduced in non-tumor areas of livers from Tlr4mut and antibiotics-treated mice (Figure 8E) suggesting that TLR4 and the intestinal microbiota provide survival signals that are advantageous for hepatocarcinogenesis.
DISCUSSION
Chronic inflammation is a key contributor to carcinogenesis in various organs including the stomach, colon, lung and liver (Balkwill and Mantovani, 2001; Grivennikov et al., 2010). In the liver, 80% of HCCs develop in a microenvironment characterized by chronic injury, inflammation and fibrosis. However, mediators that are responsible for the high risk to develop HCC in the chronically injured liver are largely unknown. Translocation of bacteria and bacterial PAMPs is common in chronic liver disease (Schuppan and Afdhal, 2008; Tandon and Garcia-Tsao, 2008), and promotes inflammation and fibrosis in chronic liver injury (Seki et al., 2007).
We now provide several lines of evidence that the intestinal microbiota and TLR4 link inflammation and carcinogenesis in the chronically injured liver. Genetic TLR4 inactivation, gut sterilization or germ-free status decrease HCC development by ≈80%. Conversely, prolonged treatment with low-dose LPS significantly increases HCC development. These findings are similar to intestinal tumorigenesis in which TLR4 and MyD88 drive carcinogenesis (Fukata et al., 2007; Lee et al., 2010; Rakoff-Nahoum and Medzhitov, 2007). In contrast to the intestine, the liver is not in direct contact with intestinal bacteria. However, the liver is the first target of intestinal bacteria and PAMPs translocating into the portal circulation. Moreover, translocation increases with the development of liver fibrosis and cirrhosis (Fukui et al., 1991; Lin et al., 1995; Wiest and Garcia-Tsao, 2005). Our HCC data in germ-free mice effectively exclude the possibility that the employed antibiotics with low systemic bioavailability might have exerted direct effects on the liver in our model. Moreover, 16s pyrosequencing demonstrated that TLR4 status and LPS treatment do not significantly change the composition of the gut microbiome with the exception of one taxon whose abundance differed between LPS- and PBS-treated mice but not between Tlr4WT and Tlr4mut mice.
In contrast to a previous study (Yu et al., 2010), we find no significant contribution of the intestinal microbiota and TLR4 to tumor initiation or purely genotoxic hepatocarcinogenesis, but demonstrate two distinct mechanisms through which the intestinal microbiota and TLR4 promote HCC: In early stages, TLR4-deficiency and gut sterilization decrease hepatic proliferation and fibrogenesis. Accordingly, gut sterilization or LPS infusion given during early stages moderately decrease or increase hepatocarcinogenesis, respectively. Our study provides evidence that TLR4-dependent HCC promotion in the early phases is predominantly mediated by TLR4-dependent secretion of growth factors such as epiregulin by hepatic stellate cells. Thus, hepatic stellate cells might provide a link between fibrosis and HCC development by increasing growth factors. Based on previous studies showing promotion of growth factor signaling and tumor development by ECM-rich and stiff environment (Levental et al., 2009; Samuel et al., 2011), it is likely that growth factors and ECM produced by hepatic stellate cells synergize in tumor promotion. Our results are consistent with recent studies showing TLR4- and MyD88/Trif-dependent regulation of epiregulin and regeneration after injury in the intestine (Brandl et al., 2010; Hsu et al., 2010). However, HCC reduction in epiregulin-deficient mice was less pronounced than in Tlr4mut or gut-sterilized mice suggesting that additional mechanisms contribute to TLR4-dependent HCC promotion.
The most significant contribution of the intestinal microbiota in our study occurred during later stages of hepatocarcinogenesis independently of CCl4-induced upregulation of growth factors and regenerative responses. These results are remarkably similar to a previous study in which NF-κB inhibition at late but not early stages reduced inflammation-associated HCC (Pikarsky et al., 2004). In line with this study, we detected decreased induction of NF-κB regulated genes and an increased rate of apoptosis in all groups that were protected from HCC development. Accordingly, we found that the hepatocyte compartment which had been unresponsive to LPS in healthy livers became highly sensitive to LPS in DEN plus CCl4-injured livers. The biological relevance of increased apoptosis for HCC development is emphasized by the strong inverse correlation of tumor number, tumor size, and liver-body weight ratio with cleaved-caspase 3-positive cells in two sets of gut-sterilized mice and in Tlr4mut mice. In addition to its role in Abcb4KO chronic liver injury model (Pikarsky et al., 2004), hepatocellular NF-κB also acts a promoter of hepatocarcinogenesis in lymphotoxin-driven HCC (Haybaeck et al., 2009) suggesting a requirement for hepatocellular NF-κB in HCC occurring in the setting of significant inflammation. In contrast, hepatocellular inactivation of NF-κB in genotoxic HCC models enhances hepatocarcinogenesis (He et al., 2010; Maeda et al., 2005). Finally, the complete but rather unphysiological absence of NF-κB activity by Nemo or TAK1 deletion causes chronic hepatocyte injury, liver cirrhosis and spontaneous HCC (Bettermann et al., 2010; Inokuchi et al., 2010; Luedde et al., 2007). Thus, the presence of inflammation and the degree of NF-κB inhibition appear to be crucial factors that determine whether NF-κB promotes or inhibits hepatocarcinogenesis.
The majority of apoptosis in our study occurred in non-tumor areas of the liver suggesting that pro-survival signals provided by the intestinal microbiota and TLR4 are required for the survival of tumor precursors, and that apoptosis prevents the development of tumor lesions rather than leading to regression of already established tumors. Accordingly, we observed no effect of gut sterilization on already established HCCs. These results are consistent with previous findings that gene expression profiles in non-tumor liver tissue, including gene sets related to NF-κB, TNFα and interferon signaling - all downstream targets of TLR4 - are crucial determinants of tumor recurrence whereas tumor profiles have no prognostic value (Hoshida et al., 2008).
One important difference between our findings and many previous studies is the tumor-promoting effect of inflammatory signals in resident liver cells. Bone marrow-derived cells such as macrophages are abundant sources of inflammatory cytokines and contribute to genotoxic HCC (Maeda et al., 2005). Our studies in chimeric mice demonstrate that BM-derived cells including hepatic macrophages do not mediate the tumor-promoting effects of TLR4 in both acute and long-term HCC models. Accordingly, we found that both hepatic stellate cells and hepatocytes are targets of LPS in the liver with TLR4 on hepatic stellate cells most likely mediating regenerative response after injury, and TLR4 on hepatocytes preventing apoptosis of hepatocytes and premalignant cells. Oval cells represent another potential target of LPS and LPS-induced mediators such as TNFα (Brooling et al., 2005), but we found only few areas with A6- and cytokeratin-positive cells typical of oval cells in our tumor model, and no influence of TLR4 status and gut-sterilization on the number these cells. Further studies in mice with conditional TLR4 deletion are required to distinguish the relative contribution of TLR4 expressed in hepatocytes versus TLR4 expressed in hepatic stellate cells to hepatocarcinogenesis.
Together with results from a previous study (Pikarsky et al., 2004), our data suggest chronic liver injury affects the resident liver compartment and makes resident liver cells and/or tumor-forming cells more dependent on inflammatory signals for their survival. With the majority of HCCs occurring in chronically injured livers but many mouse models lacking chronic injury, it is possible that the contribution of inflammatory pathway to human HCC has been underestimated. Our results differ from a previous study in which BM-derived cell populations were suggested to promote HCC through TLR4 (Yu et al., 2010). Notably, conclusions on the contribution of BM were based on acute responses to DEN instead of HCC development in that study. Moreover, TLR4- and MyD88-dependent carcinogenesis in the intestine and hepatic carcinogenesis in HCV transgenic mice are also promoted by resident cells (Hsu et al., 2010; Lee et al., 2010; Machida et al., 2009; Rakoff-Nahoum and Medzhitov, 2007). In the latter study, TLR4 promotes HCC in alcohol-treated HCV transgenic mice through the upregulation of stem cell markers. Although we observed a strong increase in the stem cell marker prominin 1/CD133 in HCCs, we observed no influence of TLR4 status on its expression in our microarray analysis suggesting that there may be additional mechanisms by which TLR4 promotes hepatocarcinogenesis in alcohol-treated HCV transgenic mice.
In order to enable bone marrow transplantation studies in adult mice, our study took advantage of the high HCC susceptibility of the C3H strain and used Tlr4WT C3H/HeOuJ and Tlr4mut C3H/HeJ mice. We can exclude that genetic differences besides TLR4 play a role in the observed promotion of HCC by TLR4 and the intestinal microbiota based on the following findings: (i) The Tlr4 locus is the only SNP among 2059 tested SNPs that differs between C3H/HeOuJ and C3H/HeJ mice (www.jax.org/phenome). (ii) Findings in C3H/HeOuJ and C3H/HeJ mice were confirmed in WT and Tlr4KO C57Bl/6 mice. (iii) A large set of experiments solely performed in C3H/HeOuJ mice such as gut sterilization and LPS infusion by osmotic pumps demonstrated the HCC-promoting role for the intestinal microbiota and the TLR4 ligand LPS.
The presented data provide conclusive evidence that the intestinal microbiota exerts a profound influence on HCC promotion in the chronically injured liver. Moreover, our study provides a strong rationale for targeting the intestinal microbiota and TLR4 for the primary or secondary prevention of HCC. The astounding reduction of HCC by gut sterilization at late stages suggests that these treatments might exert protective effects even in advanced liver disease. HCC has become the major cause of death in patients with compensated liver cirrhosis and there is a need for more effective treatment and preventative strategies (Bruix et al., 2004). To translate our findings into clinical medicine, clinically feasible methods of targeting the intestinal microbiota or TLR4 need to be established. The quadruple combination of antibiotics employed in the current study almost completely decontaminated the intestine and prevented 80% of murine HCCs, but is not suitable for long-term treatment due to known side effects in patients with advanced liver disease. Our study suggests that rifaximin, a nonabsorbable antibiotic that is widely used in patients with advanced liver disease (Bass et al., 2010), may moderately reduce HCC development. Further studies are required to determine if longer treatment duration or combination with other well-tolerated antibiotics could improve the effects of rifaximin, or if other approaches such as TLR4 antagonists would reduce HCC development.
EXPERIMENTAL PROCEDURES
Mice, HCC induction and evaluation
C3H/HeOuJ, C3H/HeJ, TLR2-deficient mice, TLR4-deficient mice (C57Bl6/10ScN), TNFR1-/IL-1R1-double deficient and C57Bl/6 mice were purchased from Jackson laboratories. Ereg-deficient mice in a C57Bl/6 background were obtained from the Mouse Mutant Regional Resource Center at the University of North Carolina.
In C3H/HeOuJ and C3H/HeJ mice, HCC was induced by intraperitoneal injection of DEN (100 mg/kg) at ages 6–14 weeks followed by 6–12 biweekly injections of carbon tetrachloride (0.5 ml/kg i.p., dissolved in corn oil) unless stated otherwise. For Ereg-deficient mice and C57Bl/6 wild-type controls, HCC was induced by the combination of DEN (25 mg/kg i.p.) given at day 15 postpartum, and 22 weekly injections of CCl4 (0.5 ml/kg i.p., dissolved in corn oil). For Tlr2KO mice and C57Bl/6 wild-type controls, HCC was induced by the combination of DEN (100 mg/kg, i.p.) given at the age of 8 weeks, followed by 25 biweekly injections of CCl4 (0.5 ml/kg, i.p., dissolved in corn oil). Gut-sterilization was done as previously described (Rakoff-Nahoum et al., 2004; Seki et al., 2007) using a combination of ampicillin (1 g/l), neomycin (1 g/l), metronidazole (1 g/l) and vancomycin (500 mg/l) in drinking water. For rifaximin studies, mice either received 250 mg/l rifaximin in drinking water with rifaximin dissolved in Ora-Plus/Ora-Sweet (Cober et al., 2010) or Ora-Plus/Ora-Sweet vehicle translating to a rifaximin dose of 50 mg/kg/d. Germ-free (GF) mouse experiments were performed at the University of North Carolina National Gnotobiotic Rodent Resource Center, Chapel Hill, North Carolina. All mice received DEN (25 mg/kg i.p.) in the germ-free status at day 15 postpartum. One week after DEN, 50% of mice were moved to a specific pathogen free (SPF) environment. 4 weeks after the DEN injection, GF and SPF mice received a total of 10 CCl4 injections (1 injection per week, at 0.5 ml/kg i.p. in corn oil). Germ-free status was confirmed by bi-weekly monitoring for the presence of bacteria in the feces by aerobic and anaerobic culture as well as Gram staining of the stool. Mice were sacrificed 30 weeks after the initial DEN injection.
Evaluation of tumor number and size was determined as described by counting the number of visible tumors and measuring the size of the largest tumor with a caliper (Naugler et al., 2007). After sacrifice, livers were explanted, digitally photographed and weighed to calculate the liver-body weight ratio. To evaluate acute effects of TLR4 and the intestinal microbiota, adult mice were treated with DEN (100 mg/kg i.p.) alone or in combination with either two or four injections of CCl4 (0.5 ml/kg i.p.), two injections of thioacetamide (400 mg/kg i.p.) or 4 weeks of a choline-deficient L-amino-acid-defined diet. For some experiments, mice were treated with lipsomomal clodronate or liposome vehicles (200 µl i.p.). For some experiments, liver fibrosis was induced by ligating the common bile duct as previously described (Kluwe et al., 2010; Seki et al., 2007). All animal procedures were in accordance to guidelines by the National Institutes of Health, and approved by the Institutional Animal Care and Use Committee at Columbia University and the Institutional Animal Care and Use Committee at the University of North Carolina, Chapel Hill.
Human samples and cells
Patients with clinical, analytical and histological features of alcoholic hepatitis were prospectively obtained in the Liver Unit of the Hospital Clínic, Barcelona, between January 2009 and December 2010 (n=10). Liver biopsies were obtained using a transjugular approach. In all patients, liver specimens were analyzed by an expert liver pathologist. Fragments of normal livers were obtained from optimal cadaveric liver donors (n=2) or resection of liver metastases (n=2). All controls had normal serum aminotransferase levels and normal liver histology. Human hepatic stellate cells were isolated by density gradient-based centrifugation from normal liver as described (Kluwe et al., 2010) and used between passage 3 and 5. Procedures involving human materials were approved by the Investigational Review Board of the Hospital Clínic of Barcelona, and informed consent was obtained from all subjects.
Primary cell isolations
Primary hepatic stellate cells, Kupffer cells and hepatocytes were isolated from either normal or DEN plus 2×CCl4-treated mice using retrograde liver perfusion and isolation techniques as previously described (Seki et al., 2007). For some experiments, hepatic stellate cells were infected with an adenovirus expressing IκB superrepressor (AdIκBsr) or an empty control adenovirus as described before (Seki et al., 2007).
SIGNIFICANCE.
Hepatocellular carcinoma (HCC) typically develops in chronically injured livers, but mediators that promote HCC in this setting are not well characterized. Our study demonstrates that TLR4 activation by LPS from the intestinal microbiota contributes to injury- and inflammation-driven tumor promotion, but not to tumor initiation. Different from most previous studies that focused on inflammatory pathways in bone marrow-derived cells, our study finds that the LPS-TLR4 pathway promotes HCC by increasing proliferative and anti-apoptotic signals in non-bone marrow-derived resident liver cells. Our finding that gut sterilization efficiently prevents HCC even at very late stages may provide a basis for clinically applicable HCC prevention strategies in patients with chronic liver injury.
Highlights.
The gut microbiota and TLR4 play a role in HCC promotion but not in HCC initiation.
Gut sterilization, germ-free status or TLR4 inactivation reduce HCC by 80–90%.
Gut sterilization efficiently suppresses hepatocarcinogenesis even when given late.
Resident liver cells but not BM-derived cells promote HCC through TLR4.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grants R01DK076920, U54CA163111 and R01AA020211 (to RFS). Germ-free studies were supported by NIH grant P40RR018603 (to RBS). D.H.D. was supported by NIH fellowship 1F31 DK091980-01. J.P. was supported by a postdoctoral fellowship from the Fondation Recherche Medicale. H.K. and R.R. were supported by R01 LM010140 and U54 CA121852. We would like to thank Drs. Ken Olive and Stephen Sastra (Columbia University) for help with ultrasound screening of tumors, Drs. Valentina Factor and Snorri Thorgeirsson (National Cancer Institute, National Institutes of Health) for providing A6 antibody, and Dr. Bo Liu (University of North Carolina) for help with GF mice. D.H.D performed animal experiments including HCC induction, antibiotics and LPS treatments, primary cell isolations, immunohistochemistry and mRNA analysis. A.M. performed HCC induction, and BMT experiments. G.G. performed animal experiments including HCC induction, antibiotics treatments, and mRNA analysis. J.P. performed immunohistochemistry, and BMT. M.J. performed acute hepatocarcinogenesis studies. I.M. performed ultrasound studies, isolations of primary cells and 16s determinations. J.M.C. performed 16s studies and immunohistochemistry. H.K. performed 16s analysis. A.A. performed studies on epiregulin secretion in hepatic stellate cells. R.B. performed studies in patients with alcoholic hepatitis. J.H.L. graded tumors. M.B. performed germ-free studies. R.F. performed statistical and functional analysis for microarray studies. R.B.S. designed germ-free studies. R.R. performed 16s analysis. R.F.S. designed and coordinated the study, performed mRNA analysis, contributed to experimental set-up, data analysis and interpretation, and drafted and edited the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers
Microarray information was deposited at the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE33446.
Supplemental Information
Supplemental Information includes 6 figures and Supplemental Experimental Procedures can be found with this article online.
REFERENCES
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Brandl K, Sun L, Neppl C, Siggs OM, Le Gall SM, Tomisato W, Li X, Du X, Maennel DN, Blobel CP, Beutler B. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci U S A. 2010;107:19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broitman SA, Gottlieb LS, Zamcheck N. Influence of Neomycin and Ingested Endotoxin in the Pathogenesis of Choline Deficiency Cirrhosis in the Adult Rat. J Exp Med. 1964;119:633–642. doi: 10.1084/jem.119.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooling JT, Campbell JS, Mitchell C, Yeoh GC, Fausto N. Differential regulation of rodent hepatocyte and oval cell proliferation by interferon gamma. Hepatology. 2005;41:906–915. doi: 10.1002/hep.20645. [DOI] [PubMed] [Google Scholar]
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, Fuster J, Garcia-Valdecasas JC, Lacy A, Suarez MJ, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Cober MP, Johnson CE, Lee J, Currie K. Stability of extemporaneously prepared rifaximin oral suspensions. Am J Health Syst Pharm. 2010;67:287–289. doi: 10.2146/ajhp090206. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt NV, Factor VM, Yasova AK, Poltoranina VS, Baranov VN, Lasareva MN. Common antigens of mouse oval and biliary epithelial cells Expression on newly formed hepatocytes. Differentiation. 1990;45:29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001;120:1251–1262. doi: 10.1053/gast.2001.23239. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D, Fukata M, Hernandez YG, Sotolongo JP, Goo T, Maki J, Hayes LA, Ungaro RC, Chen A, Breglio KJ, et al. Toll-like receptor 4 differentially regulates epidermal growth factor-related growth factors in response to intestinal mucosal injury. Lab Invest. 2010;90:1295–1305. doi: 10.1038/labinvest.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, Herdman S, Varki N, Corr M, Lee J, Raz E. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nolan JP, Leibowitz AI. Endotoxins in liver disease. Gastroenterology. 1978;75:765–766. [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rutenburg AM, Sonnenblick E, Koven I, Aprahamian HA, Reiner L, Fine J. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med. 1957;106:1–14. doi: 10.1084/jem.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Takayama H, Sharp R, Rubin JS, Merlino G, LaRochelle WJ. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, et al. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and beta-Catenin Activation to Induce Epidermal Hyperplasia and Tumor Growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni-Rugiu E, Preisegger KH, Kiss A, Audolfsson T, Shiota G, Schmidt EV, Thorgeirsson SS. Inhibition of neoplastic development in the liver by hepatocyte growth factor in a transgenic mouse model. Proc Natl Acad Sci U S A. 1996;93:9577–9582. doi: 10.1073/pnas.93.18.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Takami T, Kaposi-Novak P, Uchida K, Gomez-Quiroz LE, Conner EA, Factor VM, Thorgeirsson SS. Loss of hepatocyte growth factor/c-Met signaling pathway accelerates early stages of N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Res. 2007;67:9844–9851. doi: 10.1158/0008-5472.CAN-07-1905. [DOI] [PubMed] [Google Scholar]
- Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Komurasaki T, Uchida D, Takayama Y, Isobe T, Okuyama T, Hanada K. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, et al. Endotoxin accumulation prevents carcinogeninduced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.