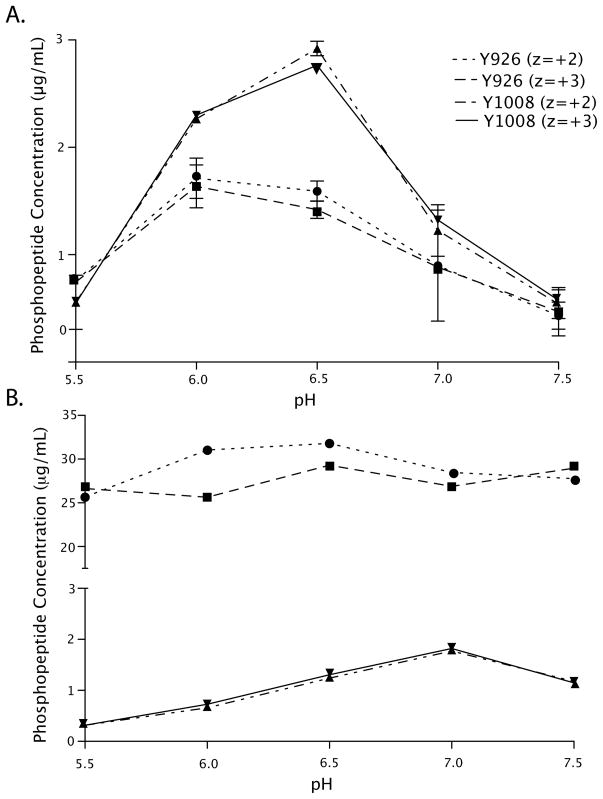
FIGURE 2.
Site-specific quantitation of phosphorylation. A. Full-length FAT domain and B. FAT domain peptides were phosphorylated in vitro with Src kinase at pH 5.5, 6.0, 6.5, 7.0, and 7.5. After phosphorylation, the full-length FAT domain was trypsinized, and the levels of the Y926 phosphorylated peptide (● z=+2, ■ z=+3) and Y1008 (▲ z=+2, ▼ z=+3) were quantified as described in the text. Comparison of the phosphorylation profiles of the full-length FAT domain and of FAT domain peptides reveals that the specificity of phosphorylation is different in the peptides than in the full-length protein. Y1008 is preferred in the full-length FAT domain whereas Y926 is preferred in the peptides. Furthermore, the pH dependence of phosphorylation of the peptides is different than that of the full-length protein.