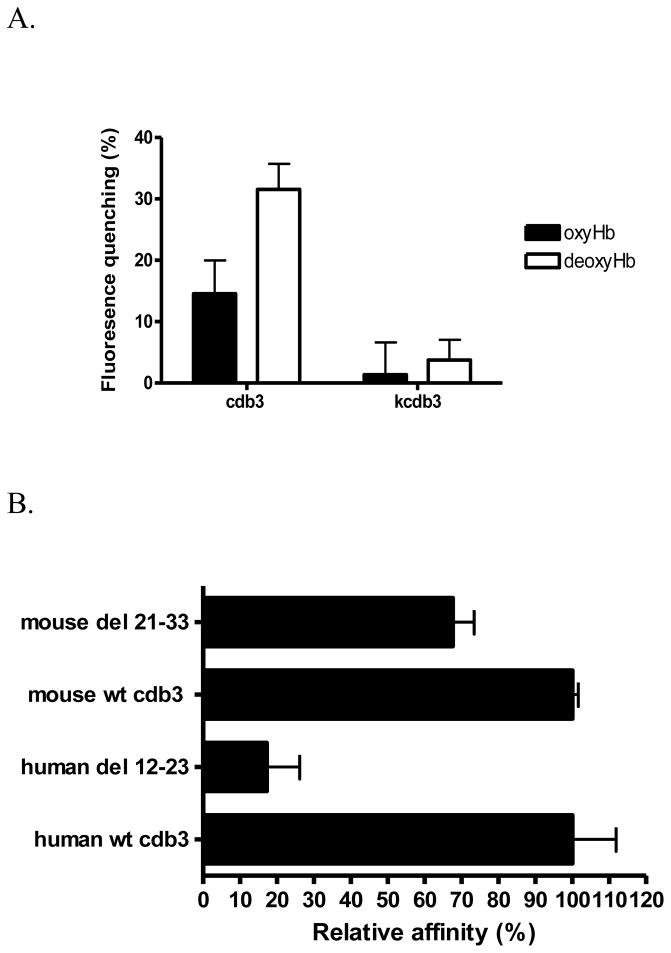
Figure 2.
A) Relative affinity of murine oxy- and deoxyHb (1μM) for murine cdb3 (0.5μM) determined by fluorescence quenching. Because the kidney spliceoform of cdb3 (kcdb3) lacks the entire NH2-terminus (residues 1-79), it was used as a nonbinding (negative) control. B) Effect of deletion from murine cdb3 of the sequence homologous to the deoxyHb binding site on human cdb3 on the affinity of the mutated murine cdb3 for murine deoxyHb. Based on the sequence alignment in panel A, the sequence in murine cdb3 (residues 21-33) found to be most homologous to the deoxyHb binding site on human cdb3 (residues 12-23) was deleted and its relative binding affinity for murine deoxyHb was examined by measuring the shift in the oxygen dissociation curve in the presence of cdb3. For comparison, the relative affinity of human deoxyHb for human cdb3 is also shown.