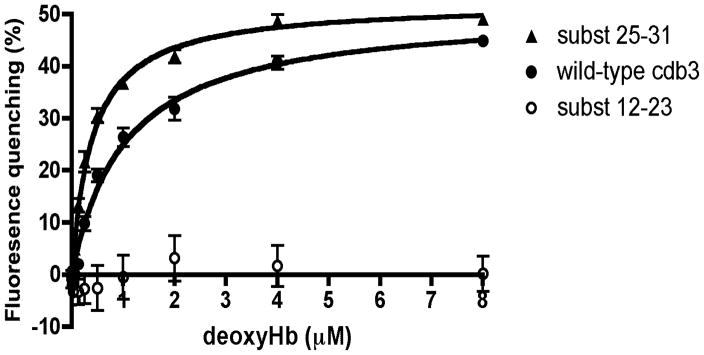
Figure 4.
Evaluation of equilibrium binding affinity of deoxyHb for various isoforms of cdb3. Increasing concentrations of deoxyHb were incubated for 45 min at room temperature with cdb3-GFP or one of its mutants and the equilibrium binding constant was calculated as described in the Methods (n=3). Derived Kd values were 1.04 ± 0.14 μM for wild type cdb3-GFP and 0.39 ± 0.03 μM for the residues 25-31 substitution mutant. The 12-23 substitution mutant displayed no measurable affinity for deoxyHb.