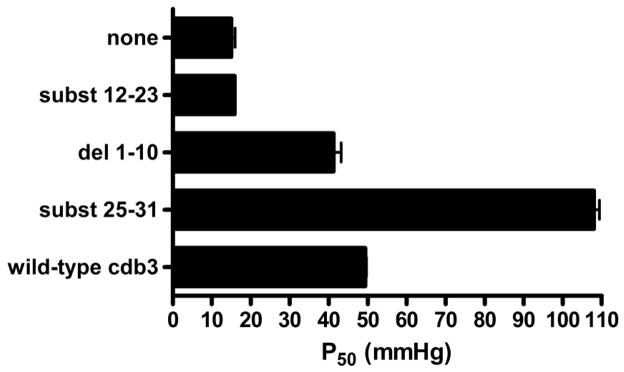
Figure 5.
Confirmation of the deoxyHb binding site on murine cdb3 by evaluation of the effect of various NH2-terminal mutants of cdb3 on the Hb-O2 dissociation curve. Hb-O2 binding curves were measured for Hb alone, or Hb in the presence of wild-type cdb3, del(1-10), del(1-21), subst(12-23), or subst(25-31), and the affinity of deoxyHb for cdb3 was determined by analysis of the oxygen pressure where the murine Hb became 50% saturated with O2 (P50).