Abstract
The treatment of metastatic renal cell carcinoma (mRCC) has recently evolved from being predominantly cytokine-based treatment to the use of targeted agents, which include sorafenib, sunitinib, bevacizumab (plus interferon alpha [IFN-α]), temsirolimus, everolimus, pazopanib, and most recently, axitinib. Improved understanding of the molecular pathways implicated in the pathogenesis of RCC has led to the development of specific targeted therapies for treating the disease. In Korea, it has been 5 years since targeted therapy became available for mRCC. Thus, we now have broader and better therapeutic options at hand, leading to a significantly improved prognosis for patients with mRCC. However, the treatment of mRCC remains a challenge and a major health problem. Many questions remain on the efficacy of combination treatments and on the best methods for achieving complete remission. Additional studies are needed to optimize the use of these agents by identifying those patients who would most benefit and by elucidating the best means of delivering these agents, either in combination or as sequential single agents. Furthermore, numerous ongoing research activities aim at improving the benefits of the new compounds in the metastatic situation or their application in the early phase of the disease. This review introduces what is currently known regarding the fundamental biology that underlies clear cell RCC, summarizes the clinical evidence supporting the benefits of targeted agents in mRCC treatment, discusses survival endpoints used in pivotal clinical trials, and outlines future research directions.
Keywords: Molecular targeted therapy, mTOR protein, Renal cell carcinoma, Vascular endothelial growth factor A
INTRODUCTION
The resistance of renal cell carcinoma (RCC) to the traditional cytotoxic chemotherapy is now well established. For many years, the mainstream therapy modality of metastatic renal cell carcinoma (mRCC) was based on cytokine-mediated approaches using either interferon alpha (IFN-α) or interleukin-2 (IL-2) or both [1]. The results with these agents were less than satisfactory because they produced objective response rates on the order of only 10 to 20%, with long-term durable responses in 5 to 7% of cases, at least for high-dose IL-2 [2,3]. Since the availability of information regarding the aberrant activities of signal transduction pathways in RCC, specific molecular targets for potential therapies have been identified and analyzed pharmacologically in a variety of in vitro and preclinical studies. As a result, the treatment of mRCC has dramatically changed in recent years. This has been driven by two groups of targeted agents: namely, vascular endothelial growth factor (VEGF)-targeted therapies and mammalian target of rapamycin (mTOR) inhibitors [4]. Since 2005, seven targeted agents have been approved by regulatory authorities in the United States (US) and Europe (Axitinib is newly approved by the US Food and Drug Administration) for various uses in advanced RCC or mRCC patients. However, despite these advancements in treatment modalities, there are many limitations when it comes to the treatment of mRCC.
The objectives of this article were to review the clinical evidence supporting the benefits of these agents, introduce the treatment guidelines, and identify limitations. Furthermore, future research directions with these targeted therapies are discussed.
EPIDEMIOLOGY OF RCC
RCC is the most common renal tumor and accounts for 3% of all adult cancers [5]. The incidence and mortality of renal malignancies have been on the rise worldwide over the past more than 30 years [6], particularly in the Western world, where kidney cancer has been among the highest of the tumors with an upward trend in incidence [6,7]. According to the recent report of the Korea Central Cancer Registry, RCC accounted for 1.8% of Korean cancers in 2008 [8]. Since then, the incidence of RCC in Korea has shown a steady increase.
Rising incidence rates are partly attributable to improvements in diagnostic imaging, but better detection does not explain the continued high number of advanced tumors and the increase in tumor size-specific mortality among RCC patients [7]. Surgery remains the mainstay therapy modality for those who present with clinically localized tumors and is an effective cure for the majority of patients. However, at least one-third of patients are diagnosed with metastases and an additional 20 to 40% of patients develop metastases after radical nephrectomy with curative intent [9-11]. The outcome of patients with mRCC is poor. The 5-year survival rate of RCC patients with metastatic lesions is 0 to 20% [12-14] and is 25 to 50% even if the metastatic lesion is solitary and can be completely resected [15-17].
IMMUNOTHERAPY FOR mRCC
Previously, systemic treatment was limited to cytokine therapy with IL-2 or IFN-α, because mRCC is largely resistant to chemotherapy [1]. Cytokine therapy is based on the rationale that stimulation of the immune system kills cancer cells. However, this modality in patients with mRCC is associated with low rates of response yet high rates of toxicity in the first-line setting [1]. In the second-line setting (in patients who have progressed on one cytokine), even fewer responses are observed, and toxicity remains similar to that of the first-line use [18]. In addition, patients' median survival period is only about 13 months [19-21].
However, high-dose IL-2 remains the only agent with proven efficacy in producing complete and partial responses in patients with mRCC [21-23]. Furthermore, despite the use of a single-agent interferon, which has decreased significantly since the introduction of targeted therapy, it remains in the frontline setting in combination with bevacizumab as a result of 2 large phase III trials [24,25]. Lastly, improved understanding of immune regulation has led to the advancement of targeted immunotherapy using immune checkpoint inhibitors that have shown promising activity and that are moving forward in clinical development [26].
MECHANISMS OF TARGETED THERAPY IN mRCC
There are at least 5 histological forms of RCC. The most prevalent is the clear cell type, which accounts for 75% of cases [27,28]. Clear cell RCC is characterized by inactivation of a crucial tumor-suppressor gene, known as von Hippel Lindau (VHL) [29,30]. Understanding the biological processes that underlie the clear cell type RCC, in particular, the central role played by the VHL-hypoxia-inducible factor (HIF)-VEGF axis, is important. This is because the various members of this cascade are the therapeutic targets for most of the agents currently used in the management of clear cell type RCC. The concept of targeting these specific signaling molecules is the fundamental underpinning of the so-called "targeted therapies." This principle has resulted in two fundamental ideologies that are nevertheless interrelated. These two principles underlie the categories of targeted therapeutics, i.e., those that block the VEGF pathway and those that block the mTOR pathway.
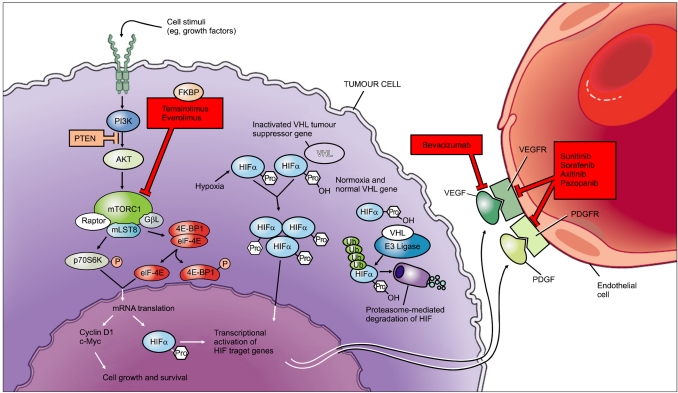
The typical mechanism of targeted therapy is shown in Fig. 1 [28]. VHL encodes the VHL protein. If VHL is inactivated, a defective VHL protein is produced and HIF is not degraded. Activated HIF then translocates into the nucleus and leads to the transcription of a wide repertoire of genes, including VEGF, platelet-derived growth factor (PDGF) [31], and transforming growth factor alpha [32], which have a central role in tumor angiogenesis and progression. Activation of the mTOR pathway also increases HIF levels [33]. This leads to increased transcription of genes, such as VEGF and PDGF, that control cell proliferation, glucose uptake, and angiogenesis [33]. Thus, increased HIF expression can promote angiogenesis in tumors.
FIG. 1.
Biological pathways and therapeutic targeted agents for renal cell carcinoma. Reprinted from Rini BI, Atkins MB, Lancet Oncol 2009;10:992-1000, with permission of Elsevier [28].
EFFICACY OF TARGETED THERAPY IN mRCC
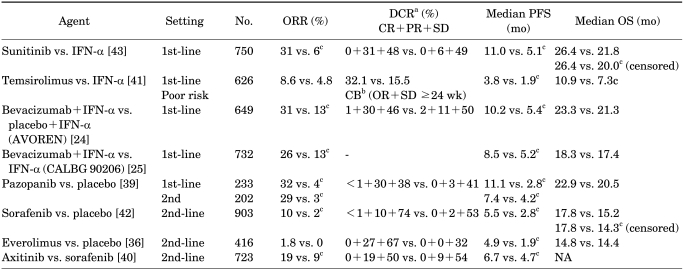
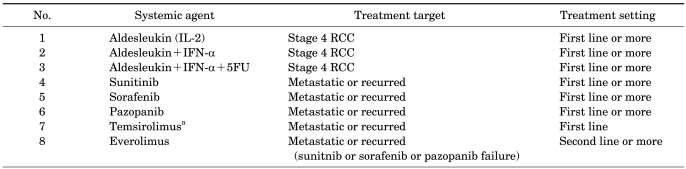
The targeted agents approved in the United States for the treatment of mRCC are sorafenib, sunitinib, bevacizumab (in combination with IFN-α), temsirolimus, everolimus, pazopanib, and, recently, axitinib. Table 1 summarizes the key efficacy data from the pivotal trials for these agents. Before the advent of targeted therapies, patients with mRCC treated with cytokines showed a median survival of 10 months [34]. Sunitinib, bevacizumab, and temsirolimus were compared with IFN-α in treatment-naïve patients and were found to be superior. In addition, sorafenib, everolimus, and pazopanib were shown to be superior to placebo in their defining trials, although the intent of many of these latter protocols was to focus on the patients who had already failed a cytokine or anti-VEGF therapy [24,25,35-39]. In a recent study, as a second-line use, axitinib was found to be superior when compared with sorafenib [40].
TABLE 1.
Targeted agents for metastatic renal cell cancer (approved): key results of phase III study
ORR, objective response rate; SD, stable disease; PFS, progression-free survival; OS, overall survival; IFN-α, interferon alpha; AVOREN, Avastin and Roferon in Renal Cell Carcinoma; CALBG, Cancer and Leukemia Group B; NA, not available.
aDCR (disease control rate), CR (complete response)+PR (partial response)+SD (stable disease) ≥3 mo, bCB, clinical benefit; OR, objective response (CR+PR), cStatistically significant.
An improvement in overall survival (OS) provides the most convincing evidence that a new therapy is superior to the existing standard modality. Only temsirolimus (for the patient with "poor-risk" RCC) had led to improved OS in randomized phase III trials [41]. However, other agents, including sunitinib, sorafenib, and pazopanib, are clearly active in RCC and constitute the comparator arm in several ongoing studies. Owing to the crossover of the patients and a widespread use of active agents in patients who progress on their assigned therapy, the OS analysis in randomized trials may be confounded. In a recent update, with censoring of crossover data, the median overall survival with sunitinib and sorafenib was significantly longer than for their respective control groups [42,43]. Therefore, the OS benefit of these drugs seems to be relatively clear.
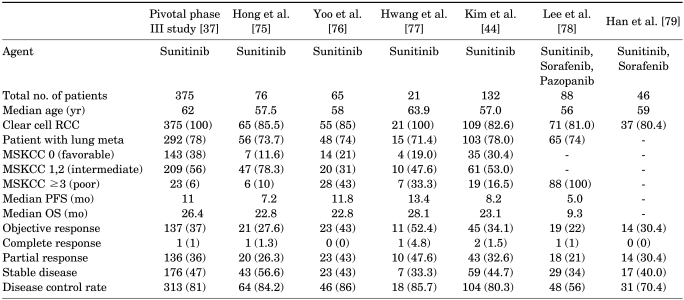
Currently, several retrospective studies and one prospective trial using tyrosine kinase inhibitors (TKIs) have been published in Korea (Table 2). According to these studies, TKIs, especially sunitinib, seem to be efficacious in a manner similar to or even better than in Western patients with slightly different treatment-related adverse events, such as a high incidence of hematological toxicities [44].
TABLE 2.
Results of targeted therapy for metastatic renal cell cancer (RCC) in Korea
Values are presented as number (%).
MSKCC, Memorial Sloan-Kettering Cancer Center risk group; PFS, progression-free survival; OS, overall survival.
CURRENT GUIDELINES FOR THE TREATMENT OF mRCC
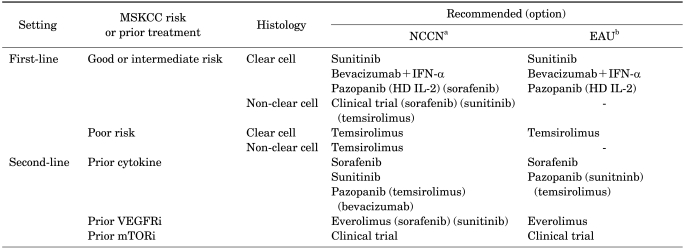
The treatment guidelines for mRCC are experiencing a rapid evolution to incorporate the new molecular-targeted therapies that have been approved recently by the US and European regulatory authorities. Table 3 summarizes the current updated recommendations contained in the National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU) guidelines.
TABLE 3.
Evidence-based clinical guidelines for systemic therapy for metastatic renal cell carcinoma
MSKCC, Memorial Sloan-Kettering Cancer Center; NCCN, National Comprehensive Cancer Network; EAU, European Association of Urology; IFN-α, interferon alpha; HD, high-dose; IL-2, interleukin-2; VEGFRi, vascular endothelial growth factor receptor inhibitor; mTORi, mammalian target of rapamycin inhibitor.
a: NCCN: Kidney Cancer, NCCN 2012 Clinical Practice Guidelines in Oncology V.1. 2012, Cat 1 & 2A, b: EAU: Guideline on Renal Cell Carcinoma 2010, Grade A.
In terms of clear cell mRCC, the NCCN guidelines (ver. 1.2012) recommend a number of options for first- and second-line treatment of mRCC. For first-line therapy, the recommended modalities for patients with a good or intermediate prognosis are sunitinib, bevacizumab plus IFN-α, and pazopanib. Temsirolimus has a recommendation for the treatment of patients with poor-prognosis mRCC. In addition, the NCCN also suggests alternatives for selected patients in the first-line setting, such as high-dose IL-2 or sorafenib, temsirolimus, and enrollment in a clinical trial. For second-line therapy, everolimus is the only agent to have an NCCN category 1 recommendation for the treatment of patients who have failed TKI treatment. Sorafenib, sunitinib, and pazopanib are recommended for the treatment of patients after cytokine failure. For the treatment of patients after the failure of other TKIs, sorafenib, sunitinib, and pazopanib are also recommended as an alternative treatment (category 2B or 3). Temsirolimus is also considered as the treatment for patients after cytokine failure (category 2A) in addition to the treatment for patients after TKI failure (category 2B). The EAU guidelines also recommend treatment options for patients with predominantly clear cell RCC. As a first-line therapy, sunitinib, bevacizumab plus IFN-α, and pazopanib are recommended for the treatment of low- and intermediate-risk patients. For the treatment of high-risk patients, temsirolimus is recommended. IFN-α monotherapy is no longer recommended. However, high-dose bolus IL-2 is recommended as a first-line treatment for mRCC in patients with clear cell histology and good prognostic factors. As a second-line therapy, everolimus has a recommendation for the treatment of patients after TKI failure; sorafenib and pazopanib are each recommended for the treatment of patients after cytokine failure; and enrollment in clinical trials is recommended for the treatment of patients after mTOR inhibitor failure.
For patients with non-clear cell mRCC, enrollment in clinical trials of systemic therapy has been the preferred strategy. However, the 2012 NCCN guidelines provide first-line therapy recommendations for patients with non-clear cell mRCC. Temsirolimus has a category 1 recommendation for the treatment of patients with poor risk non-clear cell mRCC.
In Korea, sunitinib and sorafenib have been used as a first-line treatment for patients with clear cell mRCC under the support of national health insurance since 2007. Furthermore, pazopanib has also been used since May 2011. For the treatment of non-clear cell mRCC or clear cell mRCC, with poor risk group patients, national health insurance has reimbursed the use of temsirolimus since June 2011. Most recently, everolimus was approved by the multidisciplinary committee for patients with sunitinib or sorafenib or pazopanib failure under insurance coverage. Table 4 shows the coverage of national health insurance in Korea for patients with mRCC.
TABLE 4.
Coverage of National Health Insurance for metastatic RCC in Korea
RCC, renal cell carcinoma; IL-2, interleukin-2; IFN-α, interferon alpha; 5FU, 5-fluorouracil.
a: Only for non-clear cell carcinoma or poor risk clear cell carcinoma.
DRUG RESISTANCE AND TREATMENT STRATEGIES
Some patients are inherently resistant to these targeted agents. In addition, most, if not all, patients acquire resistance over time. In general, the development of resistance has been observed after a median of 6 to 11 months of treatment [28]. Complete or durable responses have only rarely been noted, necessitating chronic therapy for most patients. Several strategies emerge from the above considerations regarding the mechanisms of resistance to the currently available therapeutics in mRCC. The first involves the use of an agent that blocks a resistance mechanism. Such an agent could be used as a monotherapy at the time of resistance to targeted therapy, could be added to continued VEGF blockade at the time of resistance, or could be added initially in combination with VEGF blockade with the hope of delaying the onset of resistance. Alternatively, more effective blockade of the initial target could involve increasing the dose of an existing agent or measuring the drug concentrations to ensure an adequate dosing, or alternatively, switching to an agent that more potently inhibits the target.
The current strategies to maximize the effectiveness of treatment are as follows.
1. Sequential therapy
Targeting different pathways may offer benefit in terms of overcoming resistance to individual agents. Sequential therapy has the potential to change mRCC into a chronic disease that can be managed for a long term through the administration of targeted agents in sequence. Although clinicians are currently using targeted agents in a sequential manner for patients with mRCC in practice, concerns remain regarding cross-resistance between the different agents. Thus, there are many questions regarding the optimal sequence for obtaining maximum clinical benefit from the available targeted therapies. In addition, adequate management of treatment-related toxicity can allow patients to remain on treatment for long periods and can help to maximize the clinical benefit of targeted agents. Recently, strategies to manage treatment-related adverse events are being refined [45].
The first randomized phase III study to investigate sequential targeted therapy in mRCC showed clinical efficacy for the sequence of sunitinib or sorafenib, followed by everolimus (RECORD-1) [36,46]. In this study, patients who had failed an earlier anti-VEGF therapy, 71% of whom had received sunitinib previously, were treated with either everolimus or placebo. The median progression-free survival (PFS) was 4.9 months vs. 1.9 months for those treated with everolimus or the placebo, respectively (hazard ratio, 0.33; 95% confidence interval, 0.25 to 0.43; p<0.01). Improvements in PFS with everolimus relative to the placebo were observed across all Memorial Sloan-Kettering Cancer Center prognostic risk groups.
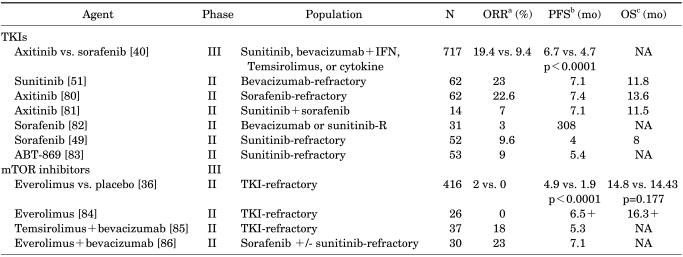
In addition, sequential TKIs are also thought to be effective. Some retrospective studies have shown that patients who fail first-line therapy with sunitinib might benefit from sorafenib treatment [47,48]. Di lorenzo et al. [49] confirmed these findings in a prospective phase II trial in which 77% of patients had failed sunitinib treatment and benefited from second-line sorafenib, with a median PFS of 16 weeks. Similar data also exist for sunitinib after sorafenib failure. Dudek et al. [50] revealed that patients who received sorafenib followed by sunitinib had a median duration of stable disease of 20 weeks (median OS, 102 weeks; 59% clinical benefit). Subsequently, it was also reported that secondline sunitinib showed efficacy in patients with an initial failure from bevacizumab [51]. The recently reported AXIS phase 3 trial directly compared the efficacy and safety of axitinib with the active comparator sorafenib in patients with mRCC who had failed one previous systemic therapy [40]. Axitinib was shown to be more effective than sorafenib in patients who had progressed after previous treatment with sunitinib, bevacizumab+IFN-α, temsirolimus, or cytokines. The AXIS trial was these patients' first exposure to a VEGFR-TKI, whereas 54% of patients had received previous sunitinib. In the subgroup of AXIS patients who had received previous sunitinib, the median PFS was 4.8 months with axitinib and 3.4 months with sorafenib (p=0.011). Furthermore, the shorter median PFS observed in both treatment arms in the sunitinib-refractory patients relative to those who received cytokines is suggestive of at least partial cross-resistance with sequential VEGF-targeted therapy. Table 5 summarizes the results of several prospective trials of sequential therapy with TKIs to TKI/mTOR inhibitors.
TABLE 5.
Prospective trials of sequential therapy (TKIs to TKIs/mTOR inhibitors)
TKIs, tyrosine kinase inhibitors; mTOR, mammalian target of rapamycin; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; INF, interferon; NA, not available.
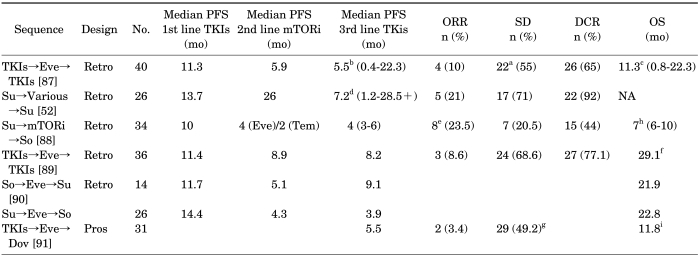
Recently, several studies evaluating the efficacy of a second VEGFR-TKI, following a VEGFR-TKI→mTOR inhibitor treatment sequence, have been reported with encouraging results (Table 6). These results suggest that reintroduction of a VEGFR-TKI following progression on a VEGFR-TKI-mTOR inhibitor treatment sequence is an effective strategy. However, patients appear to derive a lesser degree of clinical benefit from VEGFR-TKI repeat challenge than that obtained in the first-line setting, which suggests at least partial cross-resistance. Interestingly, transient resistance to the same agent has also been observed. In a recent retrospective review of 23 patients, repeat challenge with sunitinib in patients with disease progression on sunitinib and other therapies resulted in 5 patients (22%) achieving a partial response and 17 patients (74%) achieving stable disease [52]. Repeat challenge was associated with a median PFS of 7.2 months compared with 13.7 months on the initial treatment (p=0.04). The results described here indicate the potential for re-treating with an agent, despite the occurrence of resistance from the first treatment, and have implications for achieving a continuum of treatment in these patients. So far, no therapies are approved for the third-line treatment of mRCC. In clinical practice, however, a strategy that is growing is the reintroduction of a VEGFR-TKI following the progression on a VEGFR-TKI and an mTOR inhibitor.
TABLE 6.
Efficacy of treatment with a third-line TKI following treatment with sequential VEGFR-TKI→TOR inhibitor therapy
TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor; mTORi, mammalian target of rapamycininhibitor; PFS, progression-free survival; ORR, objective response rate; SD, stable disease; DCR, disease control rate; OS, overall survival; Eve, everolimus; Tem, temsirolimus; Su, sunitinb; So, sorafenib; Dov, dovitinib; Retro, retrospective; Pros, prospective.
a: SD≥3 mo, DCR: PR+SD, b: PFS: treatment of sorafenib +/-, PFS after everolimus 3.7 vs. 11.3 mo, p=0.036 in uni-variate analyses, c: OS: from start of third-line TKIs, OS: PFS first-line VEGF treatment ≥6 mo vs. <6 mo, OS 53.4 vs.19.3 mo, p=0.002, d: Median PFS: sunitinib rechallenge interval>6 mo vs. ≤6 mo, 16.5 vs. 6.5 mo, p=0.3, e: Response rate: first-line sunitinib responder vs. non-responder, 47% vs. 0%, p=0.0027, f: OS: everolimus→sunitinib vs. everolimus→sorafenib=30.5 mo vs. 17.6 mo (p=0.102), g: SD: SD≥2 mo 29 (49.2%), SD≥4 mo 16 (27.1%), h: OS: from start of sorafenib treatment, i: OS: from start of dovitinib treatment.
2. Combination therapy
The effectiveness of combination therapies is much more promising than that of single targeted therapies. It is hoped that combination therapies may induce better responses because they interfere with sequential steps in a single pathway or attack a tumor from two sides. However, for any potential clinical benefit, there must be a balance between the potential increases in toxicity associated with combining therapeutic agents. Because most combination data to date are preliminary, no combination can be said to fulfill this requirement. To determine clinical applicability, further studies are required of targeted agent combinations, if any. It is also important to consider the therapeutic options that are possible or available following the use of combination-targeted agent therapy [53].
3. Adjuvant therapy
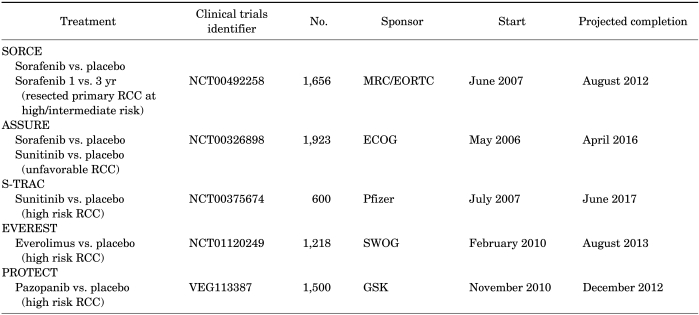
Adjuvant therapy has been used in the treatment of several malignancies with favorable results. To date, however, no medical therapies have been shown to improve outcomes in RCC when used in the adjuvant setting [54-56]. In the field of targeted therapy, the S-TRAC, ASSURE, and SORCE trials are ongoing for patients at high risk of recurrence (Table 7). The results of these trials are eagerly awaited to determine the role of targeted therapy in the adjuvant setting.
TABLE 7.
Ongoing adjuvant trials with targeted agents in renal cell carcinoma (RCC)
SORCE, A phase III randomised double-blind study comparing Sorafenib with placebo in patients with Resected primary renal cell carcinoma at high or intermediate risk of relapse; RCC, renal cell carcinoma; ASSURE, Adjuvant Sunitinib versus Sorafenib versus Placebo in Patients with Resected Renal Cell Carcinoma; S-TRAC, Sunitinib Treatment of Renal Adjuvant Cancer; EVEREST, EVErolimus for Renal Cancer Ensuing Surgical Therapy, A phase III study; PROTECT, Patient Related Outcomes with Endeavor versus Cypher Standing Trial; MRC, Medical Research Council; EORTC, European Organization for Research and Treatment of Cancer; ECOG, Eastern Cooperative Oncology Group; SWOG, Southwest Oncology Group; GSK, Glaxo Smith Kline.
4. Neoadjuvant (presurgical) therapy
Nowadays, we have yet to understand how targeted agents can be integrated with surgical approaches to maximize clinical benefit [57-60]. Cytokines do not affect the primary tumor. However, targeted therapies have an effect on the primary tumor, even in the absence of metastatic disease.
In locally advanced non-mRCC, Karakiewicz et al. [61] reported an atrial tumor thrombus that was downstaged to the level of the infrahepatic vena cava after two cycles of sunitinib. However, another study showed that only 4 of 17 patients with advanced RCC taking sunitinib, without a previous nephrectomy, experienced partial responses in their primary tumors and 1 patient progressed [62]. Neoadjuvant targeted therapy can only downstage or improve the resectability of the primary tumor or associated lesions in 20 to 25% of patients [63]. To date, there are no established criteria for discriminating between patients who will and those who will not benefit from neoadjuvant setting.
Furthermore, it is not known which patients with mRCC might benefit from an upfront targeted therapy followed by cytoreductive nephrectomy [57,64]. To clarify these critical issues, several studies are ongoing. The CARMENA and EORTC trials will provide evidencebased information regarding the benefits and the timing of cytoreductive nephrectomy in the targeted therapy era. Also in Korea, the Korean Urological Oncology Society recently led a prospective trial of "neoadjuvant sunitinib treatment for metastatic clear cell RCC" (NCT01069770).
Although studies have demonstrated the general tolerability of targeted agents, data are still limited on the safety of surgical resection following treatment with these agents, and several studies have shown increased perioperative complications after treatment [65,66].
5. Targeted therapy for non-clear cell RCC
Temsirolimus represents the only targeted therapy for which superior efficacy was confirmed in advanced non-clear cell RCC in a prospective and randomized fashion. In addition, Hudes et al. [67] demonstrated better responses in patients with advanced non-clear cell RCC than in patients with clear cell disease in their temsirolimus data, which suggests that temsirolimus is an excellent firstline option in patients with non-clear cell mRCC. Despite the absence of prospective data for the efficacy of sunitinib or sorafenib, several investigators have demonstrated the efficacy of these two agents in patients with non-clear cell mRCC [68-70]. These data indicate that targeted therapies are clearly effective in patients with non-clear cell mRCC. Therefore, we need to look at the results of large prospective studies in the future.
PRESENT AND FUTURE OF TARGETED THERAPY IN mRCC
All seven agents (sunitinib, sorafenib, temsirolomus, bevacizumab+IFN-α, pazopanib, everolimus, and axitinib) have shown efficacy and safety in phase III randomized controlled clinical trials. However, mRCC patients have to undergo chronic treatment, because targeted therapies rarely achieve durable and complete remissions. Drug resistance is the underlying reason for the growth and spread of tumors in the presence of systemic treatment. Furthermore, it remains the main barrier against long-term tumor control. In general, better tolerance of the targeted therapies than of chemotherapies is experienced by cancer patients. Nevertheless, the targeted agents still act as cytotoxic substances in a broader sense and can, therefore, cause several side effects that must not be neglected [71,72].
One therapy modality is not likely to benefit all patients, but rather a therapy modality is indicated on an individual, case-by-case basis. Treatment should be tailored to meet individual circumstances and needs, and achieving this is a considerable clinical challenge. Thus, many investigators emphasize the value of clinical judgment and experience to support treatment decisions for an individual [73]. Most patients today will receive several targeted therapies in a treatment sequence. Before starting first-line therapy, the whole potential therapeutic sequence should be considered for the individual patient.
An optimal balance between quality of life and prolongation of survival will only be achieved by considering both the benefits and the risks of the new targeted therapies [74]. We must overcome several treatment challenges to maximize the potential of targeted agents, and these include the identification of predictive molecular markers, drug resistance, the identification of the most effective sequence or combination of targeted agents, efficient clinical trial design, and the provision of cost-effective access to treatment for all patients with mRCC.
The development of targeted agents has substantially improved the prognosis of patients with mRCC. To further these advances, the following assignments are proposed for the future [53]. Identifying and optimizing the most appropriate sequence or combination of agents should be considered first. Second, overcoming drug resistance through newer agents or sequential or combination therapies should be addressed. Third, imaging techniques as predictive markers for efficacy should aim to minimize the treatment time for those patients without response to a particular agent. Fourth, molecular biomarkers should be developed to better identify the patients who are likely to benefit from a particular agent. Fifth, appropriate clinical trial designs and statistical methods to test new therapies should be developed.
CONCLUSIONS
Since the advent of targeted therapies, the modality of mRCC treatment has changed and the OS for these patients is now greater than 2 years in prospective studies. Currently, the first-line standard of care for patients with clear cell mRCC includes sunitinib, bevacizumab combination with IFN-α, pazopanib, and temsirolimus, all of which are options for patients with highrisk characteristics. Everolimus has proven efficacy as a second-line targeted therapy. The sequential use of targeted therapies can improve PFS and OS. Additional progression-free survival benefits might be derived from cytoreductive nephrectomy while we await for the results of ongoing phase III trials. However, we have much work to be completed. As more information regarding mechanisms of disease and drug resistance becomes available, new targets, new targeted agents, and new combinations will be studied with the goal of providing maximal efficacy with manageable toxicity.
ACKNOWLEDGEMENTS
This study was supported by a Korean National Cancer Center Grant, No. 1110560.
Footnotes
The authors have nothing to disclose.
References
- 1.Oudard S, George D, Medioni J, Motzer R. Treatment options in renal cell carcinoma: past, present and future. Ann Oncol. 2007;18(Suppl 10):x25–x31. doi: 10.1093/annonc/mdm411. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148–154. [PubMed] [Google Scholar]
- 3.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 4.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(Suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 6.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 8.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 10.Linehan WM, Walther MM, Alexander RB, Rosenberg SA. Adoptive immunotherapy of renal cell carcinoma: studies from the Surgery Branch, National Cancer Institute. Semin Urol. 1993;11:41–43. [PubMed] [Google Scholar]
- 11.Mevorach RA, Segal AJ, Tersegno ME, Frank IN. Renal cell carcinoma: incidental diagnosis and natural history: review of 235 cases. Urology. 1992;39:519–522. doi: 10.1016/0090-4295(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 12.Ficarra V, Righetti R, Pilloni S, D'amico A, Maffei N, Novella G, et al. Prognostic factors in patients with renal cell carcinoma: retrospective analysis of 675 cases. Eur Urol. 2002;41:190–198. doi: 10.1016/s0302-2838(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 13.McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–1095. doi: 10.1016/s0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 15.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 16.Kierney PC, van Heerden JA, Segura JW, Weaver AL. Surgeon's role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1:345–352. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 17.Tongaonkar HB, Kulkarni JN, Kamat MR. Solitary metastases from renal cell carcinoma: a review. J Surg Oncol. 1992;49:45–48. doi: 10.1002/jso.2930490111. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Chevreau C, Lasset C, Douillard JY, Ravaud A, Fabbro M, et al. Groupe Français d'Immunothérape. Cytokines in metastatic renal cell carcinoma: is it useful to switch to interleukin-2 or interferon after failure of a first treatment? J Clin Oncol. 1999;17:2039–2043. doi: 10.1200/JCO.1999.17.7.2039. [DOI] [PubMed] [Google Scholar]
- 19.Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;(1):CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 21.Sun M, Lughezzani G, Perrotte P, Karakiewicz PI. Treatment of metastatic renal cell carcinoma. Nat Rev Urol. 2010;7:327–338. doi: 10.1038/nrurol.2010.57. [DOI] [PubMed] [Google Scholar]
- 22.Di Lorenzo G, Buonerba C, Biglietto M, Scognamiglio F, Chiurazzi B, Riccardi F, et al. The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev. 2010;36(Suppl 3):S16–S20. doi: 10.1016/S0305-7372(10)70015-3. [DOI] [PubMed] [Google Scholar]
- 23.Singer EA, Gupta GN, Srinivasan R. Update on targeted therapies for clear cell renal cell carcinoma. Curr Opin Oncol. 2011;23:283–289. doi: 10.1097/CCO.0b013e32834479c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 25.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George S, Pili R, Carducci MA, Kim JJ. Role of immunotherapy for renal cell cancer in 2011. J Natl Compr Canc Netw. 2011;9:1011–1018. doi: 10.6004/jnccn.2011.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantuck AJ, Zisman A, Belldegrun A. Biology of renal cell carcinoma: changing concepts in classification and staging. Semin Urol Oncol. 2001;19:72–79. [PubMed] [Google Scholar]
- 28.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10:992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 29.Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22:200–209. doi: 10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 31.Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990;86:670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A, et al. Role of transforming growth factor-alpha in von Hippel--Lindau (VHL)(-/-) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:1387–1392. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 35.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 38.Shariat SF, Karam JA, Karakiewicz PI. Words of wisdom. Re: Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ; Global ARCC Trial. N Engl J Med 2007;356:2271-81. Eur Urol. 2009;55:250–252. doi: 10.1016/j.eururo.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 40.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 41.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 42.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 43.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Hong MH, Kim K, Shin SJ, Ahn JB, Jeung HC, et al. Sunitinib for Asian patients with advanced renal cell carcinoma: a comparable efficacy with different toxicity profiles. Oncology. 2011;80:395–405. doi: 10.1159/000330361. [DOI] [PubMed] [Google Scholar]
- 45.Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol. 2008;53:917–930. doi: 10.1016/j.eururo.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 46.Calvo E, Escudier B, Motzer RJ, Oudard S, Hutson TE, Porta C, et al. Everolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer. 2012;48:333–339. doi: 10.1016/j.ejca.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Sablin MP, Negrier S, Ravaud A, Oudard S, Balleyguier C, Gautier J, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182:29–34. doi: 10.1016/j.juro.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 48.Tamaskar I, Garcia JA, Elson P, Wood L, Mekhail T, Dreicer R, et al. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J Urol. 2008;179:81–86. doi: 10.1016/j.juro.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 49.Di Lorenzo G, Cartenì G, Autorino R, Bruni G, Tudini M, Rizzo M, et al. Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. J Clin Oncol. 2009;27:4469–4474. doi: 10.1200/JCO.2009.22.6480. [DOI] [PubMed] [Google Scholar]
- 50.Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 51.Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 52.Zama IN, Hutson TE, Elson P, Cleary JM, Choueiri TK, Heng DY, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer. 2010;116:5400–5406. doi: 10.1002/cncr.25583. [DOI] [PubMed] [Google Scholar]
- 53.Gore ME, Larkin JM. Challenges and opportunities for converting renal cell carcinoma into a chronic disease with targeted therapies. Br J Cancer. 2011;104:399–406. doi: 10.1038/sj.bjc.6606084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kjaer M, Iversen P, Hvidt V, Bruun E, Skaarup P, Bech Hansen J, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285–289. doi: 10.3109/00365598709180784. [DOI] [PubMed] [Google Scholar]
- 55.Margulis V, Matin SF, Tannir N, Tamboli P, Shen Y, Lozano M, et al. Randomized trial of adjuvant thalidomide versus observation in patients with completely resected high-risk renal cell carcinoma. Urology. 2009;73:337–341. doi: 10.1016/j.urology.2008.08.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messing EM, Manola J, Wilding G, Propert K, Fleischmann J, Crawford ED, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21:1214–1222. doi: 10.1200/JCO.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Richey SL, Culp SH, Jonasch E, Corn PG, Pagliaro LC, Tamboli P, et al. Outcome of patients with metastatic renal cell carcinoma treated with targeted therapy without cytoreductive nephrectomy. Ann Oncol. 2011;22:1048–1053. doi: 10.1093/annonc/mdq563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–66. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Culp SH, Tannir NM, Abel EJ, Margulis V, Tamboli P, Matin SF, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378–3388. doi: 10.1002/cncr.25046. [DOI] [PubMed] [Google Scholar]
- 60.You D, Jeong IG, Ahn JH, Lee DH, Lee JL, Hong JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. 2011;185:54–59. doi: 10.1016/j.juro.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Karakiewicz PI, Suardi N, Jeldres C, Audet P, Ghosn P, Patard JJ, et al. Neoadjuvant sutent induction therapy may effectively down-stage renal cell carcinoma atrial thrombi. Eur Urol. 2008;53:845–848. doi: 10.1016/j.eururo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 62.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Bex A, de Gast G, Haanen JB, et al. Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res. 2008;14:2431–2436. doi: 10.1158/1078-0432.CCR-07-4089. [DOI] [PubMed] [Google Scholar]
- 63.Wood CG, Margulis V. Neoadjuvant (presurgical) therapy for renal cell carcinoma: a new treatment paradigm for locally advanced and metastatic disease. Cancer. 2009;115(10 Suppl):2355–2360. doi: 10.1002/cncr.24240. [DOI] [PubMed] [Google Scholar]
- 64.Choueiri TK, Garcia JA, Elson P, Khasawneh M, Usman S, Golshayan AR, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 65.Jonasch E, Wood CG, Matin SF, Tu SM, Pagliaro LC, Corn PG, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–4081. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas AA, Rini BI, Stephenson AJ, Garcia JA, Fergany A, Krishnamurthi V, et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009;182:881–886. doi: 10.1016/j.juro.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Hudes GR, Berkenblit A, Feingold J, Atkins MB, Rini BI, Dutcher J. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36(Suppl 3):S26–S36. doi: 10.1053/j.seminoncol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 69.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 70.Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH, Jr, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–1280. doi: 10.1002/cncr.24864. [DOI] [PubMed] [Google Scholar]
- 71.Di Lorenzo G, Porta C, Bellmunt J, Sternberg C, Kirkali Z, Staehler M, et al. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59:526–540. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Ravaud A. Treatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapies. Oncologist. 2011;16(Suppl 2):32–44. doi: 10.1634/theoncologist.2011-S2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009;7:618–630. doi: 10.6004/jnccn.2009.0043. [DOI] [PubMed] [Google Scholar]
- 74.Kirchner H, Strumberg D, Bahl A, Overkamp F. Patient-based strategy for systemic treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10:585–596. doi: 10.1586/era.10.25. [DOI] [PubMed] [Google Scholar]
- 75.Hong MH, Kim HS, Kim C, Ahn JR, Chon HJ, Shin SJ, et al. Treatment outcomes of sunitinib treatment in advanced renal cell carcinoma patients: a single cancer center experience in Korea. Cancer Res Treat. 2009;41:67–72. doi: 10.4143/crt.2009.41.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoo C, Kim JE, Lee JL, Ahn JH, Lee DH, Lee JS, et al. The efficacy and safety of sunitinib in korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol. 2010;40:980–985. doi: 10.1093/jjco/hyq073. [DOI] [PubMed] [Google Scholar]
- 77.Hwang E, Lee HJ, Sul CK, Lim JS. Efficacy and safety of sunitinib on metastatic renal cell carcinoma: a single-institution experience. Korean J Urol. 2010;51:450–455. doi: 10.4111/kju.2010.51.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JL, Park I, Park K, Park S, Ahn Y, Ahn JH, et al. Efficacy and safety of vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma and poor risk features. J Cancer Res Clin Oncol. 2012;138:687–693. doi: 10.1007/s00432-012-1148-8. [DOI] [PubMed] [Google Scholar]
- 79.Han KS, Jung DC, Choi HJ, Jeong MS, Cho KS, Joung JY, et al. Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer. 2010;116:2332–2342. doi: 10.1002/cncr.25019. [DOI] [PubMed] [Google Scholar]
- 80.Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 81.Dutcher JP, Wild D, Hudes GR, Stadler WM, Kim S, Tarazi JC, et al. Sequential axitinib (AG-013736) therapy of patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib alone [abstract 5127]; Poster presented at the 44th Annual Meeting of the American Society of Clinical Oncology; 2008 30 May-3 June; Chicago, Illinois, USA. Alexandria: American Society of Clinical Oncology; 2008. [Google Scholar]
- 82.Garcia JA, Hutson TE, Elson P, Cowey CL, Gilligan T, Nemec C, et al. Sorafenib in patients with metastatic renal cell carcinoma refractory to either sunitinib or bevacizumab. Cancer. 2010;116:5383–5390. doi: 10.1002/cncr.25327. [DOI] [PubMed] [Google Scholar]
- 83.Tannir N, Wong Y, Kollmannsberger C, Ernstoff MS, Perry DJ, Appleman LJ, et al. Phase II trial of ABT-869 in advanced renal cell cancer (RCC) after sunitinib failure: efficacy and safety results [abstract] J Clin Oncol. 2009;27(15S) Meeting abstract no. 5036. [Google Scholar]
- 84.Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115:2438–2446. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 85.Merchan JR, Pitot HC, Qin R, Liu G, Fitch TR, Picus J, et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): safety and activity of RKI refractory RCC patients [abstract] J Clin Oncol. 2009;27(15 Suppl) Meeting abstract no. 5039. [Google Scholar]
- 86.Hainsworth JD, Spigel DR, Burris HA, 3rd, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28:2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]
- 87.Grünwald V, Seidel C, Fenner M, Ganser A, Busch J, Weikert S. Treatment of everolimus-resistant metastatic renal cell carcinoma with VEGF-targeted therapies. Br J Cancer. 2011;105:1635–1639. doi: 10.1038/bjc.2011.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Lorenzo G, Buonerba C, Federico P, Rescigno P, Milella M, Ortega C, et al. Third-line sorafenib after sequential therapy with sunitinib and mTOR inhibitors in metastatic renal cell carcinoma. Eur Urol. 2010;58:906–911. doi: 10.1016/j.eururo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Blesius A, Beuselinck B, Chevreau C, Ravaud A, Rolland F, Oudard S, et al. Are TKIs still active in patients treated with TKI and everolimus? Experience from 36 patients treated in France in the RECORD 1 trial. Ann Oncol. 2010;21(Suppl 8):viii271–viii303. doi: 10.1016/j.clgc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Porta C, Paglino C, Procopio G, Sabbatini R, Bellmunt J, Schmidingers M, et al. Optimizing the sequencial treatment of metastatic renal cell carcinoma (MRCC) - a retrospective, multicenter, analysis of 40 patients treated with either sorafenib, an mTOR Inhibitor (mTORI) and sunitinib, or sunitinib, an mTORI and sorafenib. Eur J Cancer. 2011;47(Suppl 1):S514. [Google Scholar]
- 91.Angevin E, Grunwald V, Ravaud A, Castellano DE, Lin CC, Gschwend JE, et al. A phase II study of dovitinib (TKI258), an FGFR- and VEGFR-inhibitor, in patients with advanced or metastatic renal cell cancer (mRCC) [abstract] J Clin Oncol. 2011;29(15 Suppl) Meeting abstract no. 4551. [Google Scholar]