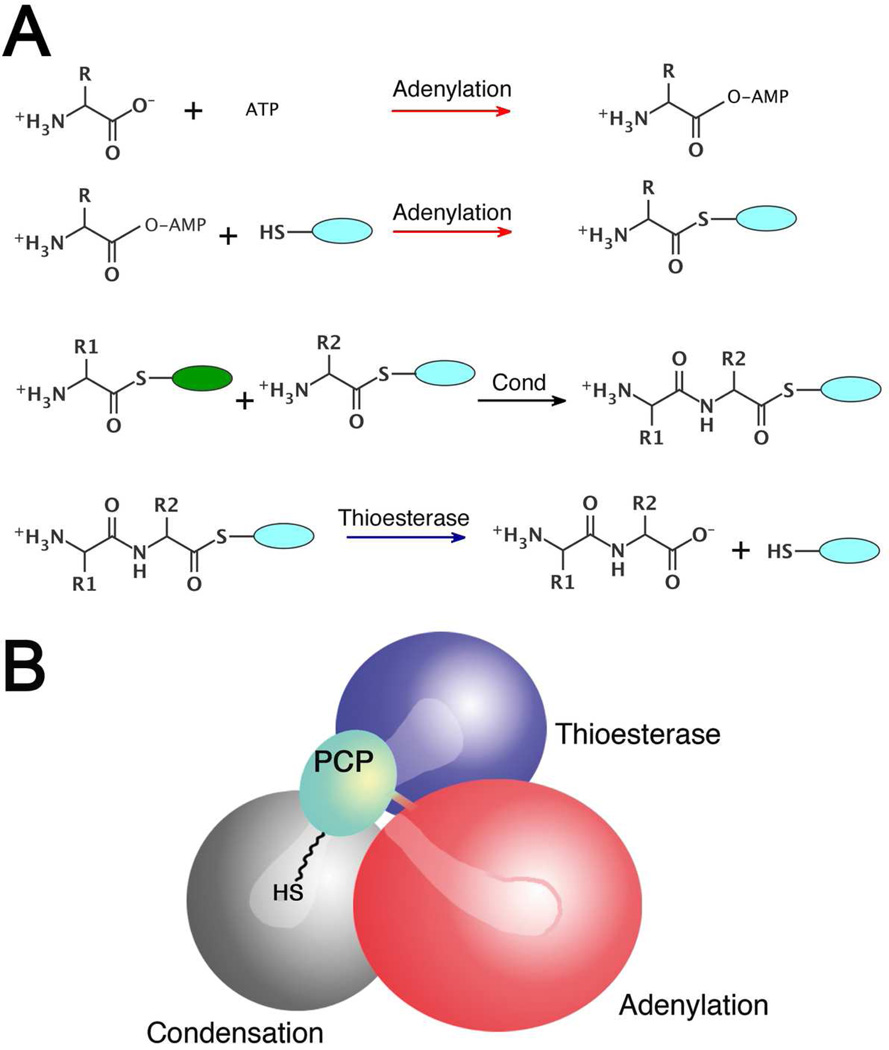
Figure 1.
Multidomain catalysis of peptide synthesis by the NRPSs. (A) Reactions catalyzed by the most common NRPS catalytic domains. The adenylation domain catalyzes a two step reaction to first adenylate the amino acid and then covalently load the downstream carrier domain, represented by the blue oval. The pantetheine cofactor is represented by the thiol SH. The domain alternation hypothesis suggests a 140° rotation of a small C-terminal subdomain within the adenylation domain is used to adopt the catalytic conformations for the two partial reactions. The condensation domain catalyzes peptide bond formation, transferring the loaded amino acid from an upstream carrier domain (green) to form the dipeptide on the downstream carrier (blue). The terminal thioesterase domain catalyzes thioester hydrolysis, releasing the peptide. (B) Schematic representation of SrfA-C highlighting the structural problem of the domain rearrangements required to enable the phosphopantetheine cofactor to reach the neighboring active sites. The schematic represents the domain orientation observed in the SrfA-C structure (PDBID:2VSQ). The condensation domain is grey, the adenylation domain is red, the PCP domain is light blue, and the thioesterase is dark blue.