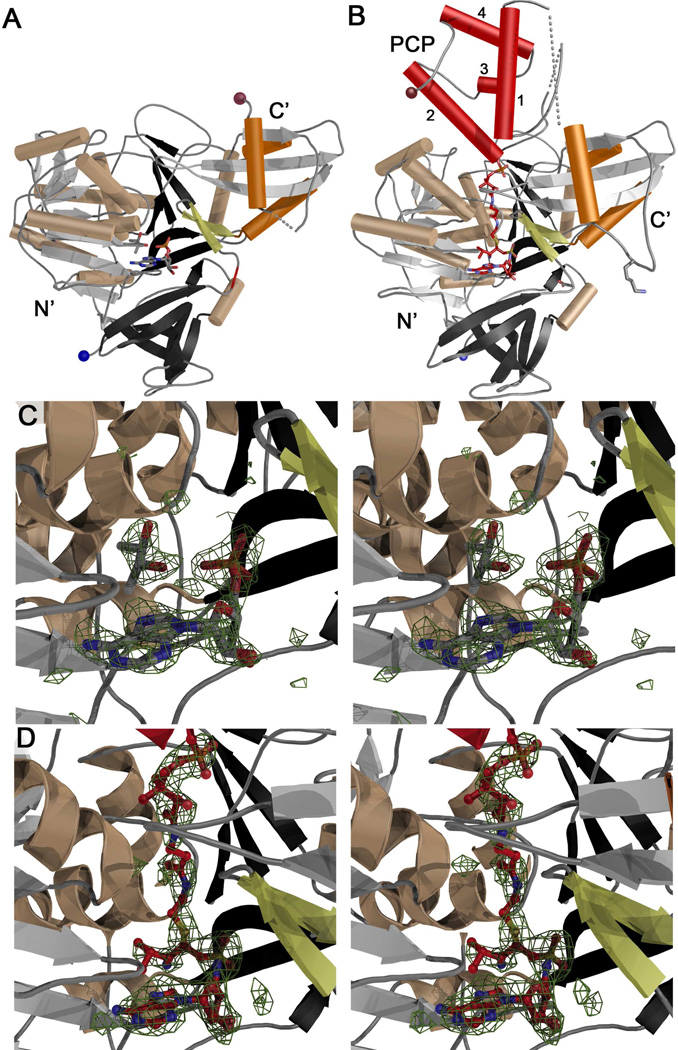
Figure 3.
Structures of apo- and holo-PA1221. (A) Ribbon diagram of apo-PA1221 with N-terminal domain colored grey, black, and wheat, the C-terminal subdomain highlighted with orange helices. The A8 loop, an antiparallel sheet that immediately follows the hinge at Asp417, is shown in yellow and the N- and C-termini are indicated with blue and brown spheres. (B) Ribbon diagram of holo-PA1221, colored as in panel A with the PCP colored red and the four helices labeled 1–4. Lys499, the catalytic lysine from the A10 motif, is shown in stick representation. The same loop, Ala496-Leu500, is disordered in the apo-structure and is shown with a dotted line in panel A. (C) Stereo image of apo-PA1221 active site with AMP and (2R,3R)-(−)-butanediol and Fo-Fc difference density contoured at 3 σ. (D) Stereo image of holo-PA1221 phosphopantetheine tunnel and active site with phosphopantetheine and Val-AVS with Fo-Fc difference density contoured at 3 σ. Both electron density maps were created with coefficients calculated prior to inclusion of ligands in the model.