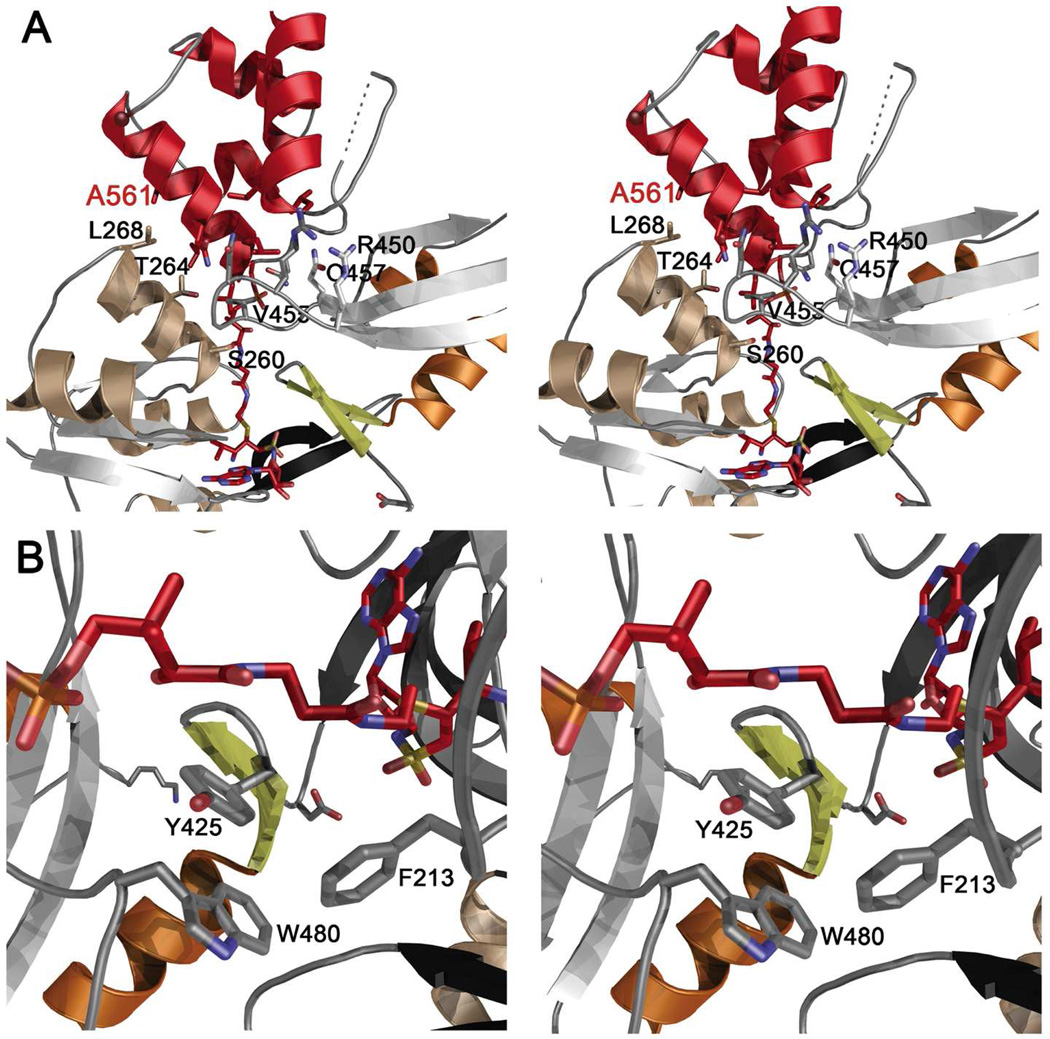
Figure 4.
Stereorepresentations of holo-PA1221. (A) Ribbon diagram of the interaction between the Adenylation and PCP domains. The PCP is shown in red, while the adenylation domain is colored as in Figure 3B. Several residues from the adenylation domain are labeled in black to orient the viewer. The PCP residue Ala561 is labeled red; this residue is located on helix 2. The residues that form the hydrophobic interface with this helix include Thr264 and Leu268. Leu 261 and Leu265 are shown but not labeled for clarity. Val455 is shown interacting with Leu554 and Leu555, which are not labeled. Residues that contribute to the hydrogen bonding network include Arg450, Arg452, Asn453, and Gln457 are shown. These residues interact primarily with main chain atoms of the PCP. (B) Phe213, the conserved aromatic residue of the A4 motif, is stabilized by interactions with aromatic residues Tyr425 and Trp480 of the C-terminal domain. Rotation of Phe213 opens the phosphopantetheine tunnel allowing proper binding of the pantetheine for thioester formation.