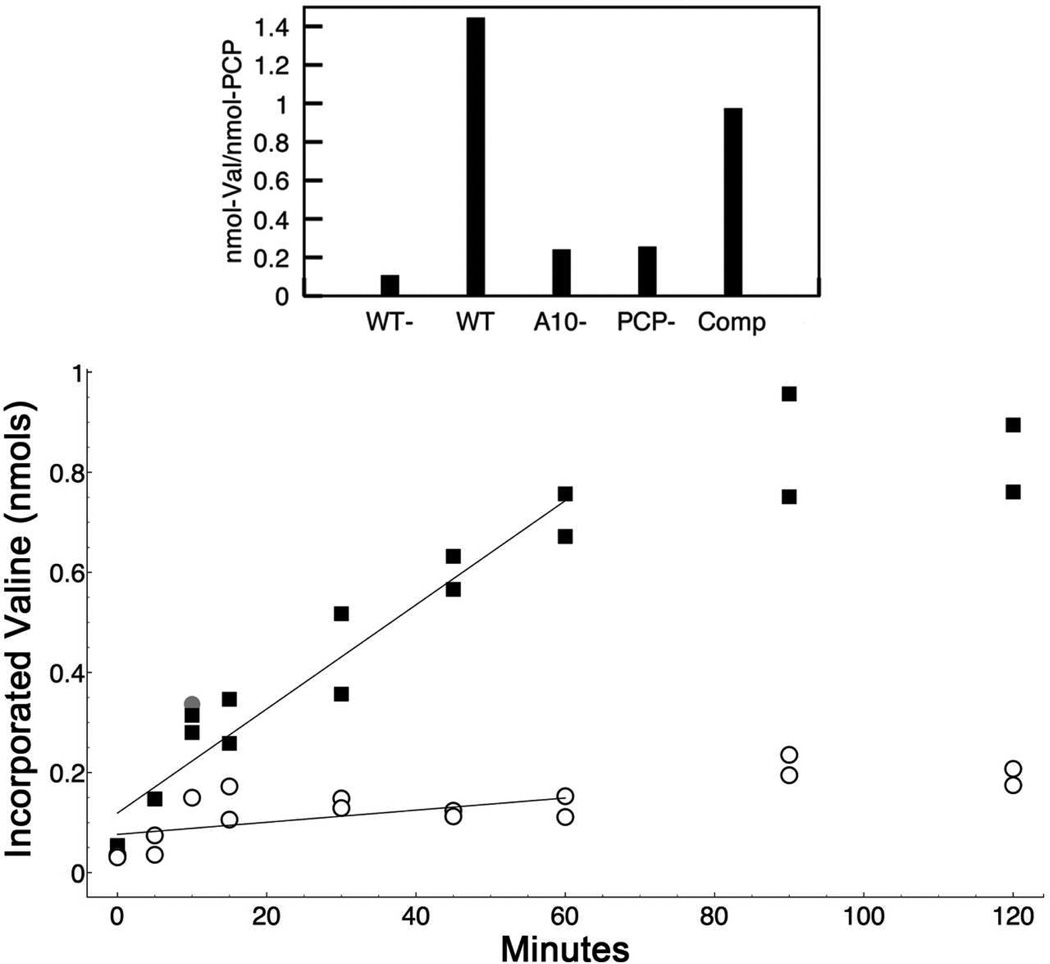
Figure 5.
Functional analysis of intra- vs. inter-molecular loading with the PA1221 protein. The top panel shows the initial loading of 3H-Valine onto PA1221 at 37°C. The individual bars represent averages of two assays with wild-type enzyme in the absence (WT-) and presence (WT) of ATP, the K499L mutant (A10-), the S553A mutant (PCP-) and a co-incubation reaction containing equal amounts of the K499L and the S553A mutant enzymes (Comp). Results are expressed as nmol valine incorporated per nmol of functional PCP as the experiment with the compensatory mutants used equal amounts of total protein, or half as much of each functional domain. In the bottom panel, the reaction was monitored on ice, slowing the reaction to observe differences between the wt and the combination of the K499L and S553A mutant enzymes. Charging with 3H-valine was monitored over two hours. Loading of the WT enzyme is represented by the filled squares. The inter-molecular reaction, forced through the reaction containing equal amounts of the two compensatory mutants, K499L and S553A, is represented by the open circle. All data points, reflecting duplicate reactions at each time point, are shown. The filled grey circle is an anomalous data point from the compensatory mutant experiment that was omitted from the linear regression plot to better represent the rate of incorporation. Results are expressed as nmol of valine incorporated into the 3 nmol of holo-PCP domain used in both experiments.