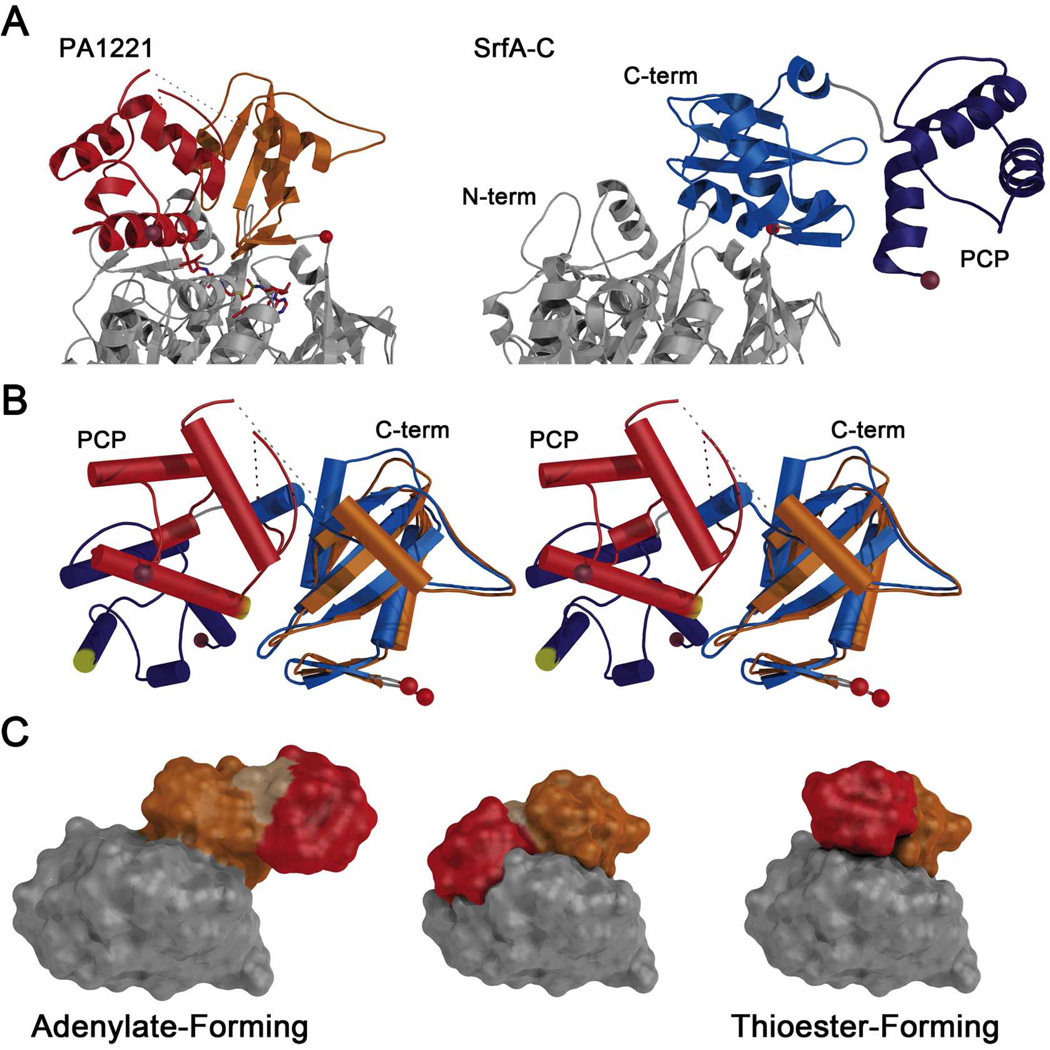
Figure 7.
Comparison of holo-PA1221 with SrfA-C adenylation and PCP domains. (A) The PA1221 and SrfA-C proteins were aligned on the basis of the N-terminal subdomain for comparison of the overall organization of C-terminal subdomains and PCPs. Where the (left) PA1221 carrier domain packs against the adenylation domains, the SrfA-C protein (right) extends the PCP to interact with the neighboring condensation domain. (B) The SrfA-C (blue) and PA1221 (orange and red) C-terminal subdomain and PCPs were aligned on the basis of the central sheet in the C-terminal subdomain. A stereo representation illustrates the differences in rotations of the PCP domain relative to the C-terminal subdomain. The pantetheine attachment site at the start of helix 2 of the PCP is highlighted in yellow. (C) Schematic model for the role of domain alternation in the movement of modular PCPs. Each panel illustrates the SrfA-C adenylation and PCP domains. The N- and C-terminal subdomains are grey and orange; the PCP is red. The adenylate-forming model is the orientation of the experimentally determined adenylation and PCP domains from 2VSQ. The middle model was created by superposing the C-terminal subdomain and PCP of 2VSQ as a rigid body onto PA1221, using the C-terminal subdomains for alignment. In this model, the PCP and N-terminal subdomains overlap, illustrating that a second component of rotation is needed to adopt the assumed thioester-forming model (right panel), which was created by superimposing both the SrfA-C C-terminal subdomain and the PCP onto the similar domain orientation in PA1221.