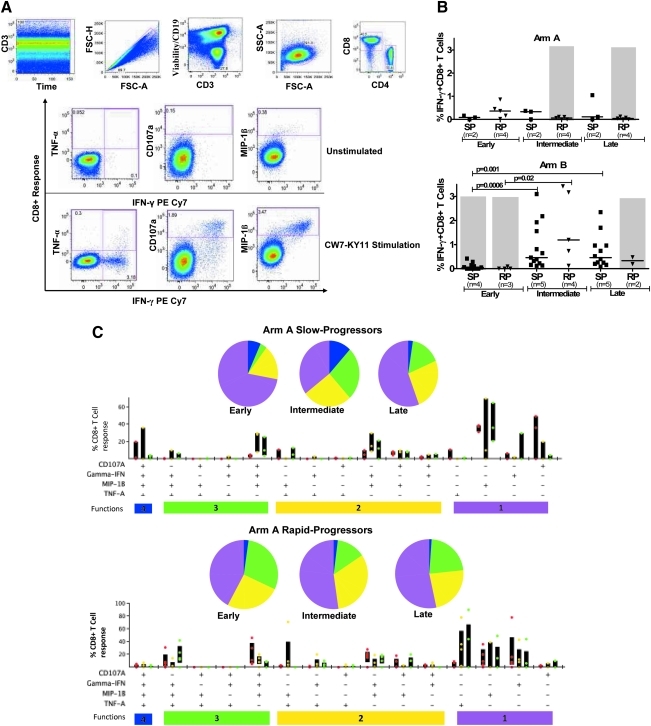
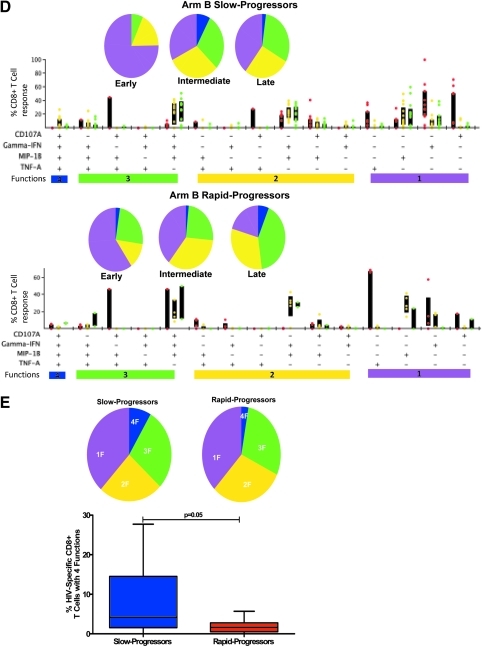
FIG. 1.
Measurement of HIV-1-specific CD8+ T cell functionality by multicolor staining in slow-progressor and rapid-progressor children. (A) Representative gating scheme for identification of polyfunctional HIV-1-specific CD8+ T cells following stimulation with HIV-1 peptide is shown for one individual. Initial gating was on the CD3 versus time to eliminate random laser events, followed by removal of doublets by forward scatter height (FSC-H) versus area (FSC-A). Live/dead and B cells were discriminated by the use of viability dye and anti-CD19, respectively, versus CD3; subsequently the lymphocyte population was gated, followed by identification of CD8+ and CD4+ T cells. Gates for each of the measured four CD8+ T cell functions were set based on the negative control (unstimulated, media only). Boolean gating was used to create a full array of the 16 possible response patterns when testing four functions. (B) Longitudinal intracellular measurement of interferon (IFN)-γ expression by CD8+ T cells. Measurement of CD8+ IFN-γ+ CD8+ T cells by intracellular cytokine staining (ICS) in arm A (top panel) and arm B (bottom panel) slow (SP) and rapid-progressor (RP) children throughout the first 3–4 years of life. Dot plots show median values of the different epitopes (as shown in Table 2) targeted by different individuals in each group. The shaded area in gray shows time on antiretroviral therapy (ART). (C, D) Representative functional composition of HIV-1-specific CD8+ T cell responses in slow-progressor and rapid-progressor children. The functional flavor of the individual response patterns to IFN-γ, MIP-1β, CD107a, and TNF-α is denoted by colored dots; red (early), yellow (intermediate), and green (late time points) for arm A (C) and arm B (D) children. The proportion contributed by the response pattern from slow-progressor (top panel) and rapid-progressor (bottom panel) individuals and the interquartile ranges are shown. The response patterns for each individual and the epitopes studied (as depicted in Table 2) were grouped and color coded by the number of functions and summarized in pie chart form where each slice of the pie represents the fraction of the total epitope-specific response that consist of CD8+ T cells with the respective number of functions. (E) Summarized comparison of the overall proportions of polyfunctional CD8+ T cells in slow-progressor and rapid-progressor children. Top panel: Polyfunctionality as depicted on the pie charts was obtained by grouping slow-progressors (left pie chart) and rapid-progressors (right pie chart) at comparable time points while not on ART. For arm A this was the early time point (slow progressors, n of individuals=2, n of epitopes=3; rapid progressors, n of individuals=4, n of epitopes=5) and for arm B this was the intermediate time-point (slow progressors, n of individuals=4, n of epitopes=13; rapid progressors, n of individuals=4, n of epitopes=5). The pie charts represent the medians of the different functions for the two groups at the stipulated time points. Bottom panel: The contribution of HIV-1-specific CD8+ T cells with four functions to the overall virus-specific CD8+ T cell response was compared between the combined slow (n of individuals=7, n of epitopes=16) and rapid (n of individuals=8, n of epitopes=10) progressor children.