Abstract
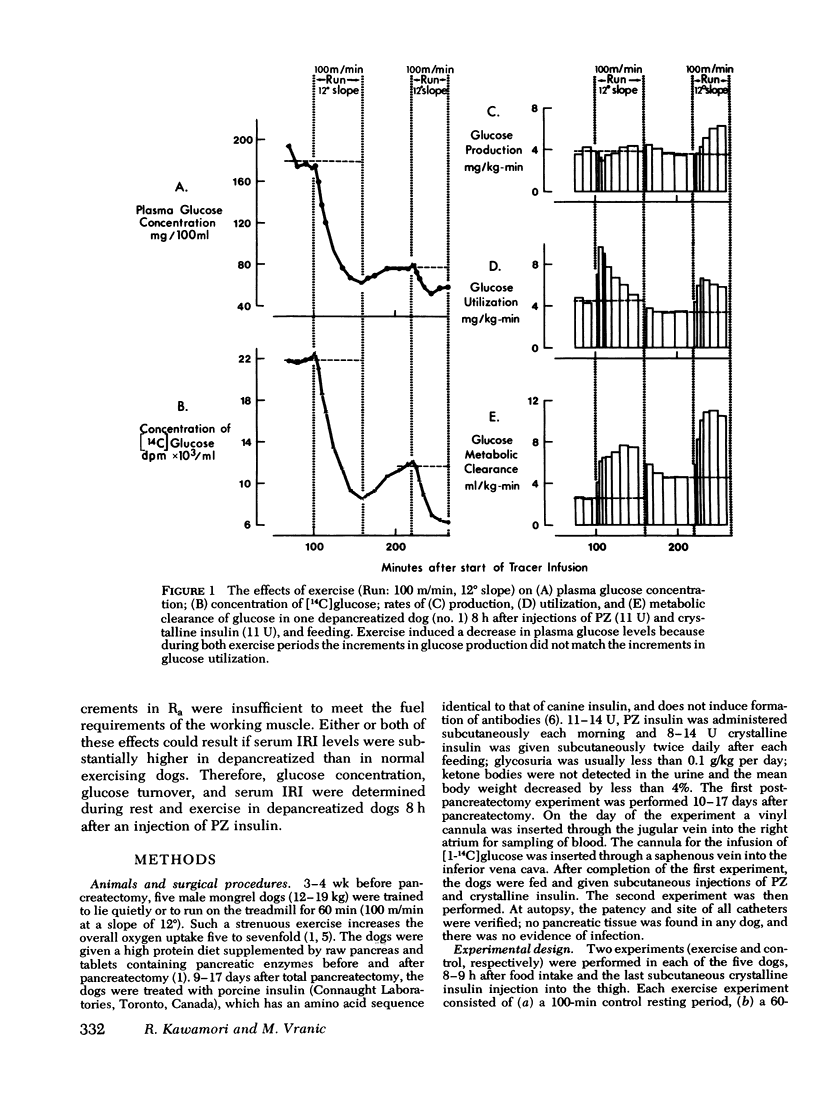
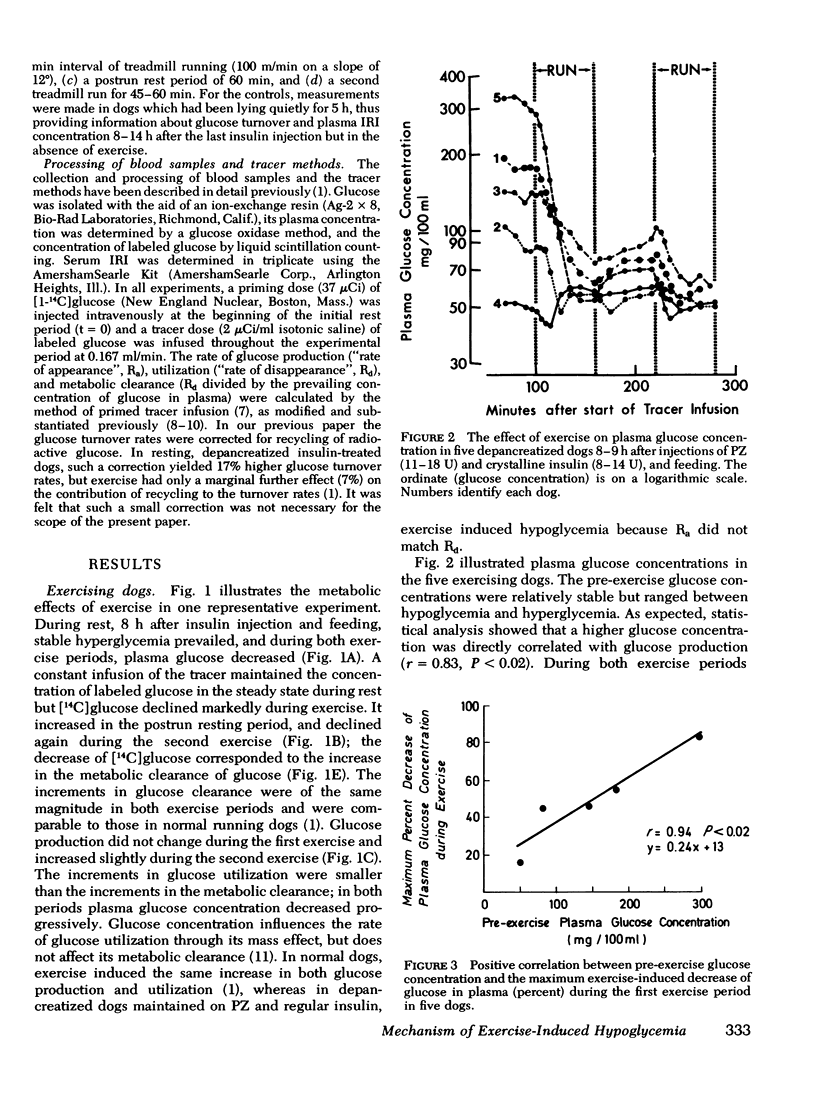
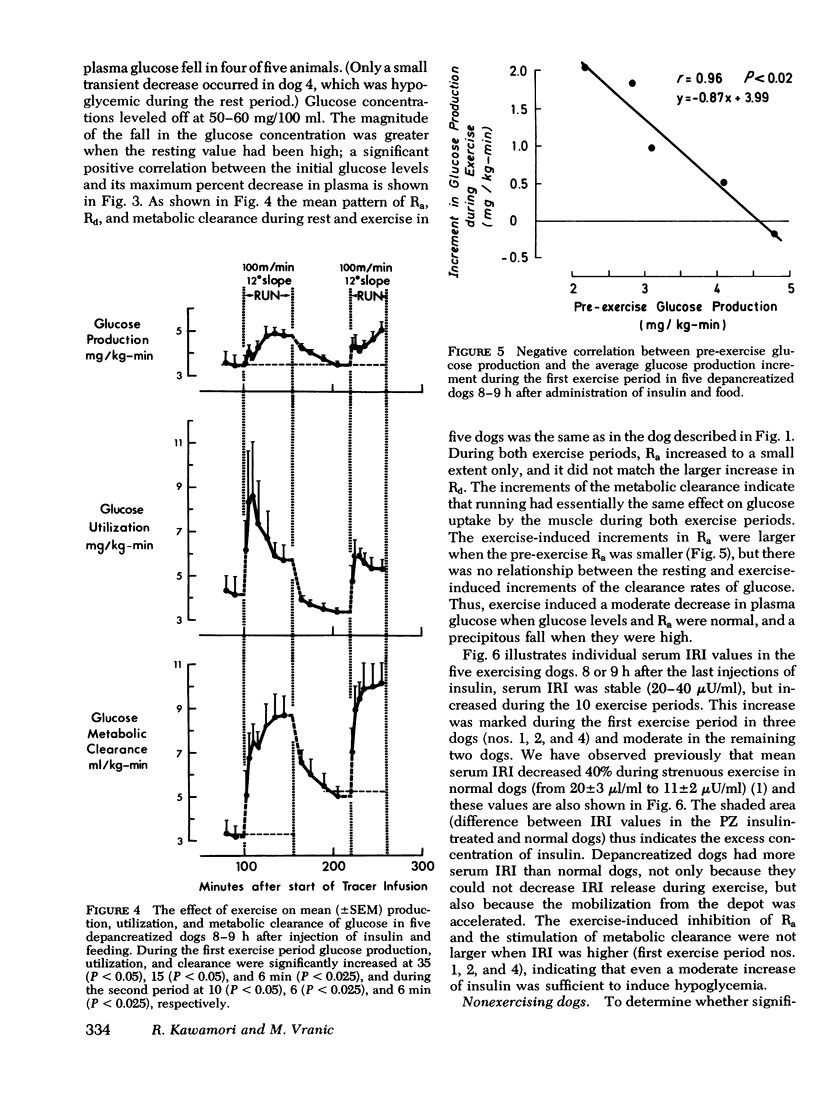
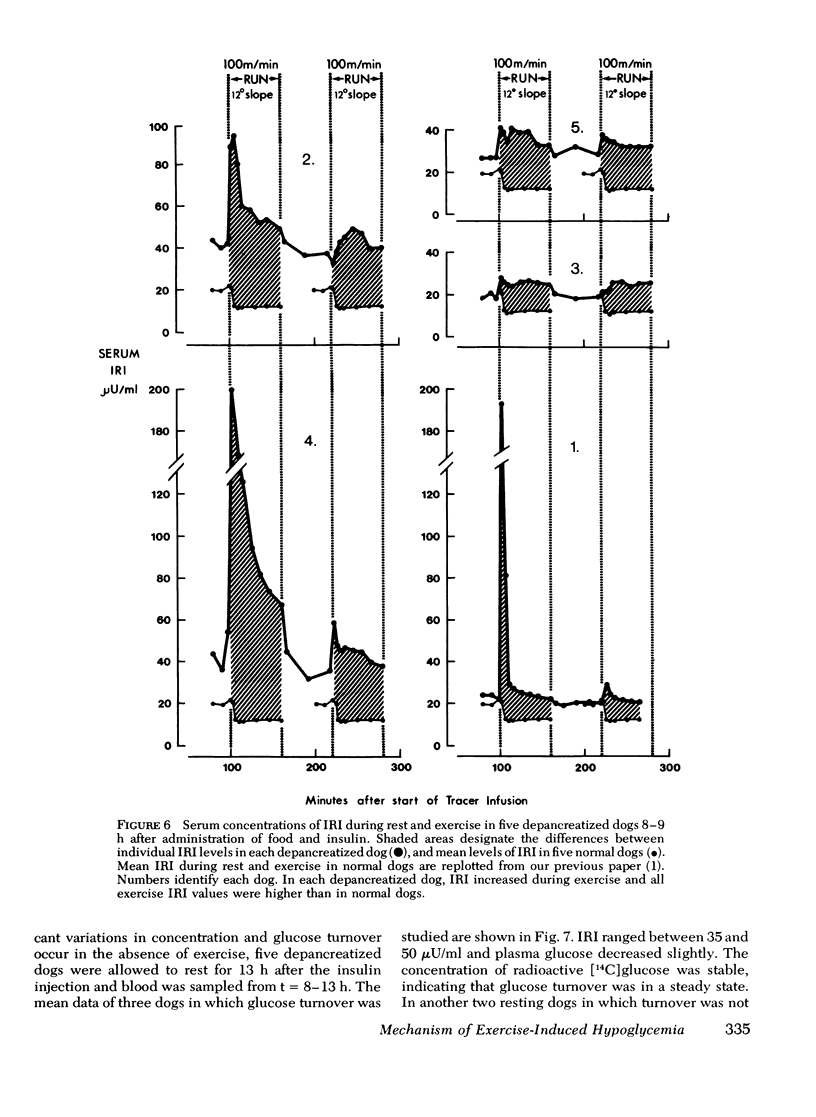
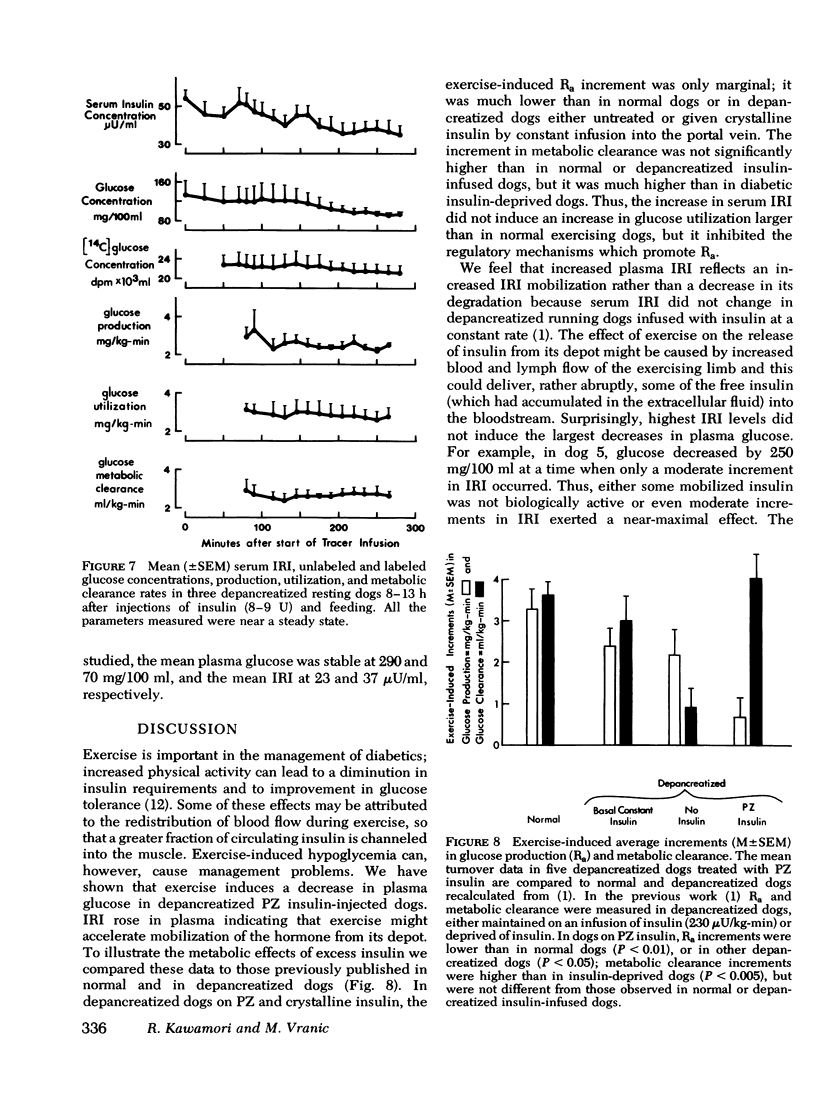
Human diabetics on intermediate and long-acting insulin occasionaly become hypoglycemic during exercise. We have shown previously that during exercise, hypoglycemia did not occur in depancreatized insulin-infused dogs because the increments in glucose production and utilization were proportional and of the same magnitude as in normal dogs. Therefore, to elucidate the mechanism of the glucose-lowering effect of strenuous exercise, we measured glucose production and utilization, metabolic clearance of glucose, and serum immunoreactive insulin in postabsorptive depancreatized dogs 8 h after a subcutaneous injection of protamine zinc and crystalline insulin. During rest, plasma glucose was stable, but ranged between hypoglycemia and hyperglycemia. Hyperglycemia was associated with overproduction of glucose, indicating insulin deficiency despite normal or elevated serum immunoreactive insulin. Glucose clearance, as in normal dogs, increased threefold but glucose production increased only marginally (50%) and, consequently, glucose decreased in plasma. The decrease of plasma glucose was directly proportional to the preexercise concentration and production of glucose. The magnitude of inhibition glucose production was not correlated with the serum immunoreactive insulin indicating either that some released insluin was not active or that a moderate immunoreactive insulin increment induced a near-maximal inhibition. It is concluded that in depancreatized dogs injected with protamine zinc insulin, exercise accelerates mobilization of insulin from its injection site presumably because of increased blood and lymph flow. Glucose utilization did not exceed that in normal dogs, but hepatic glucose production failed to increase sufficiently to meet the needs of muscle in exercise.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Hagg S., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Interaction of insulin and exercise on glucose uptake. Biochem J. 1975 Jan;146(1):231–238. doi: 10.1042/bj1460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973 Jul;22(7):537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of interaction between insulin and glucagon on glucose turnover and FFA concentration in normal and depancreatized dogs. Metabolism. 1974 Aug;23(8):729–744. doi: 10.1016/0026-0495(74)90005-5. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Felig P., Wahren J. Fuel homeostasis in exercise. N Engl J Med. 1975 Nov 20;293(21):1078–1084. doi: 10.1056/NEJM197511202932107. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I. Metabolism in normal and pancreatectomized dogs during steady-state exercise. Am J Physiol. 1967 Oct;213(4):857–862. doi: 10.1152/ajplegacy.1967.213.4.857. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- SANDERS C. A., LEVINSON G. E., ABELMANN W. H., FREINKEL N. EFFECT OF EXERCISE ON THE PERIPHERAL UTILIZATION OF GLUCOSE IN MAN. N Engl J Med. 1964 Jul 30;271:220–225. doi: 10.1056/NEJM196407302710502. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R., Pek S., Kovacevic N., Wrenshall G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous exercise in dogs. J Clin Invest. 1976 Feb;57(2):245–255. doi: 10.1172/JCI108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]


