Fig. 1.
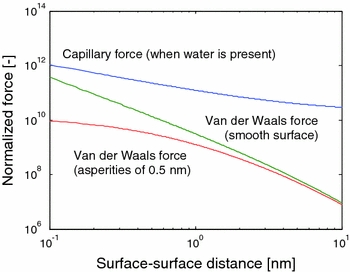
The main forces between two silica particles of 10 nm as a function of the interparticle distance. All forces are normalized by dividing them by the gravity forces on a single particle. The capillary force is given for water; for other liquids, this force is typically lower. The van der Waals force depends on the surface roughness, as shown by the curves for a smooth surface and for surface asperities. Models and constants from Butt and Kappl (2010)
