Abstract
The ability of two common protected forms of amines (carbamates and sulfonamides) to serve as directing groups in nickel-catalyzed Suzuki reactions has been exploited in the development of catalytic asymmetric methods for cross-coupling unactivated alkyl electrophiles. Racemic secondary bromides and chlorides undergo carbon–carbon bond formation in a stereoconvergent process in good ee at room temperature in the presence of a commercially available nickel complex and chiral ligand. Structure-enantioselectivity studies designed to elucidate the site of binding to nickel (the oxygen of the carbamate and of the sulfonamide) led to the discovery that sulfones also serve as useful directing groups for asymmetric Suzuki cross-couplings of racemic alkyl halides. To the best of our knowledge, this investigation provides the first examples of the use of sulfonamides or sulfones as effective directing groups in metal-catalyzed asymmetric carbon–carbon bond-forming reactions. A mechanistic study established that transmetalation occurs with retention of stereochemistry and that the resulting nickel–carbon bond does not undergo homolysis in subsequent stages of the catalytic cycle.
Exploiting a “directing” group within a substrate to provide a transient two-point interaction with a chiral reagent/catalyst is a powerful strategy for enantioselective synthesis, since chelation decreases conformational flexibility and can facilitate the effective transmission of stereochemical information from the reagent/catalyst to the substrate.1 Recently, we have reported the first examples of asymmetric cross-couplings of unactivated alkyl electrophiles, processes that are directed by an aromatic ring, the oxygen of a carbonyl group, or the nitrogen of an arylamine. 2,3 Expanding the scope of enantioselective couplings to other families of functionalized electrophiles is an important objective. In this report, we demonstrate that nickel-catalyzed asymmetric alkyl–alkyl Suzuki reactions can be directed by a diverse array of functional groups, specifically, carbamates, sulfonamides, and sulfones (eq 1); to the best of our knowledge, these represent the first examples of the use of sulfonamides and sulfones as directing groups in metal-catalyzed enantioselective carbon–carbon bond-forming processes.
In earlier studies, we determined that racemic secondary halides that bear a proximal arylamine (1) undergo asymmetric Suzuki cross-coupling with good ee.2c Although this method furnishes access to enantioenriched amines, which are a very important class of bioactive compounds, arylamines are not as pervasive as alkylamines.4
 |
(1) |
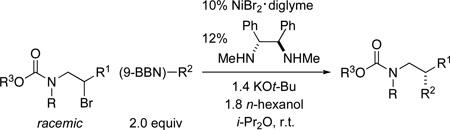
In order to access enantioenriched amines other than tertiary arylamines (1), while avoiding the use of nitrogen mustard-like substrates,5 we decided to examine carbamate-protected halo amines6 (e.g., 2) as electrophiles in stereoconvergent alkyl–alkyl Suzuki reactions. We were pleased to determine that a chiral nickel/diamine catalyst can indeed accomplish asymmetric Suzuki couplings of racemic carbamate-protected halo amines with alkylboranes (Table 1). Thus, carbamates derived from secondary dialkylamines (entries 1–3) and from secondary arylamines (entries 4 and 5) cross-couple in good ee at room temperature. The compatibility of this method with an aryl carbamate (entries 1–4) and with an aryl methyl ether (entry 2) is noteworthy, since both functional groups undergo C–O bond cleavage in the presence of other nickel-based catalysts for Suzuki cross-couplings;7 in contrast, both are inert (>95% recovery in control experiments) to our reaction conditions. The nickel source (NiBr2•diglyme) and the diamine ligand are commercially available.
Table 1.
Catalytic asymmetric Suzuki cross-couplings of unactivated alkyl electrophiles to generate carbamate-protected amines.a
 | ||||
|---|---|---|---|---|
| entry | electrophile | R2 | ee (%) | yield (%)b |
| 1 |  |
91 | 56 | |
| 2 |  |
90 | 83 | |
| 3 | 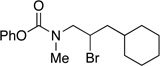 |
90 | 74 | |
| 4 | 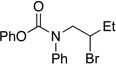 |
n-Hex | 90 | 56 |
| 5 | 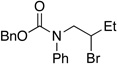 |
80 | 66 | |
All data are the average of two experiments.
Yield of purified product.
The stereochemistry of the catalyst, not of an alkoxy substituent of the carbamate, determines the stereochemical outcome of this carbamate-directed asymmetric carbon–carbon bond-forming process (eq 2 and eq 3). Furthermore, under identical conditions, a racemic secondary alkyl chloride cross-couples in good ee and yield (eq 4). Perhaps predictably, increasing the distance between the directing group and the electrophilic site leads to an erosion in enantioselectivity when the standard method is applied; however, it is clear that it will be possible to address this shortcoming through appropriate modification of the reaction conditions (eq 5). The carbamate, which serves both as a protecting group and a directing group, can be hydrolyzed in good yield to liberate the secondary amine (eq 6).
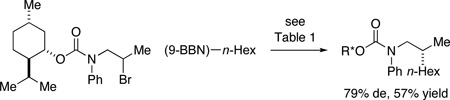 |
(2) |
 |
(3) |
 |
(4) |
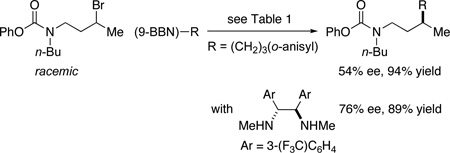 |
(5) |
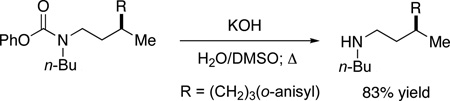 |
(6) |
To further expand access to enantioenriched amines via asymmetric cross-couplings of alkyl electrophiles, we chose to pursue sulfonamide-directed processes. The achievement of this objective would be noteworthy not only in the context of the asymmetric synthesis of amines, but also more broadly since, to the best of our knowledge, there are no examples of metal-catalyzed enantioselective carbon–carbon bond-forming reactions directed by a sulfonamide. The conditions that we had employed for couplings of carbamate-protected amines (Table 1) furnished promising results for a racemic sulfonamide, and optimization of the reaction conditions provided an enhancement in enantioselectivity and yield (Table 2; the diamine ligand is commercially available).
Table 2.
Sulfonamide-directed catalytic asymmetric Suzuki cross-couplings of unactivated alkyl electrophiles.a
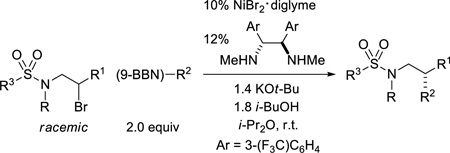 | ||||
|---|---|---|---|---|
| entry | electrophile | R2 | ee (%) | yield (%)b |
| 1 | 90 | 58 | ||
| 2 | 90 | 54 | ||
| 3 | 90 | 76 | ||
| 4 | 72 | 68 | ||
All data are the average of two experiments.
Yield of purified product.
As illustrated in Table 2, both tosyl- and mesyl-protected secondary dialkylamines are suitable cross-coupling partners, undergoing stereoconvergent carbon–carbon bond formation in good ee at room temperature (entries 1–3).8,9 A sulfonamide derived from an arylamine couples with more moderate enantioselectivity, perhaps due to the diminished Lewis basicity of the sulfonamide (entry 4). As observed for carbamate-directed asymmetric Suzuki reactions (eq 4 and eq 5), the sulfonamide-directed cross-coupling of a corresponding unactivated secondary chloride also proceeds in good ee and yield (eq 7), whereas a homologous alkyl bromide couples with modest enantioselectivity (eq 8).
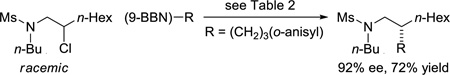 |
(7) |
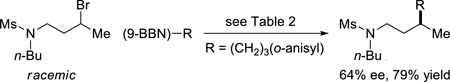 |
(8) |
In our previous studies of asymmetric cross-couplings of unactivated alkyl halides, we focused our attention on the use of alkyl nucleophiles as coupling partners. However, we have recently described modest success when an arylboron reagent was employed in an amide-directed Suzuki reaction (82% ee, 47% yield).2d In the case of sulfonamide-directed cross-couplings, for the first time we have achieved an arylation of an unactivated electrophile with excellent enantioselectivity and yield (eq 9).
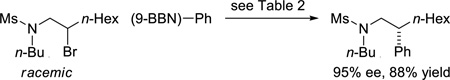 |
(9) |
Given that we have observed both nitrogen-2c and oxygen-directed2b, d asymmetric cross-couplings of unactivated alkyl halides, we sought insight into which heteroatom is the more likely binding site to nickel in these sulfonamide-directed processes. When we “reverse” the sulfonamide such that the distance between the sulfonamide oxygens and the electrophilic carbon remains virtually the same, but the nitrogen and the electrophilic carbon are now separated by two additional atoms, the cross-coupling nevertheless proceeds with good enantioselectivity (eq 10). In view of our previous observations that the distance between the directing group and the electrophilic site generally has a significant impact on ee (e.g., eq 5 and eq 8), we believe that the sulfonamide oxygen is likely binding to nickel in the asymmetric Suzuki reactions illustrated in Table 2.
 |
(10) |
Further support for the suggestion of oxygen as the site of complexation is provided by our observation that unactivated secondary alkyl electrophiles that bear sulfones also undergo stereoconvergent carbon–carbon bond formation in good ee; both alkyl bromides (Table 3)10 and alkyl chlorides (eq 11)11 are suitable coupling partners. To the best of our knowledge, these are the first examples of sulfone-directed, metal-catalyzed enantioselective carbon–carbon bond-forming processes.
Table 3.
Sulfone-directed catalytic asymmetric Suzuki cross-couplings of unactivated alkyl electrophiles.a
 | ||||
|---|---|---|---|---|
| entry | electrophile | R2 | ee (%) | yield (%)b |
| 1 |  |
88 | 79 | |
| 2 | 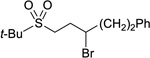 |
87 | 81 | |
| 3 | 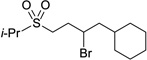 |
90 | 84 | |
All data are the average of two experiments.
Yield of purified product.
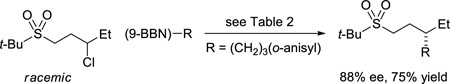 |
(11) |
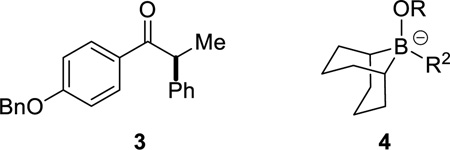
Although KOt-Bu is employed as a stoichiometric additive in the Suzuki couplings described above, the conditions are not highly Brønsted-basic. For example, when enantioenriched ketone 3 is included in a reaction mixture, it can be recovered at the end of the cross-coupling with virtually no erosion in enantiomeric excess. In contrast, in the absence of the trialkylborane, the ketone is completely racemized. By 11B NMR spectroscopy, we have determined that an “ate” complex (4) is generated under the cross-coupling conditions, which significantly attenuates the Brønsted basicity of the reaction medium.12
Our current hypothesis is that these asymmetric cross-couplings of secondary alkyl electrophiles follow the pathway depicted in Figure 1.13 In order to gain insight into the transmetalation process, which is likely the turnover-limiting step in these Suzuki reactions,14 we examined the cross-coupling of an unactivated secondary halide with a diastereomerically enriched alkyl-(9-BBN) reagent under the conditions described in Table 2 (eq 12 and eq 13).15,16 Analysis of the appropriate coupling constants revealed that the alkyl group of the organoborane is transferred to the reaction product with retention of configuration at carbon, consistent with transmetalation with retention, as well with structural integrity for the Ni–R2 bond (e.g., toward homolytic cleavage) during the catalytic cycle.17,18
Figure 1.
Outline of a mechanism for nickel-catalyzed enantioselective Suzuki cross-couplings of unactivated secondary alkyl halides.
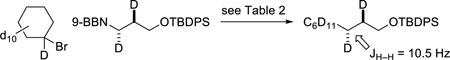 |
(12) |
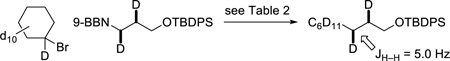 |
(13) |
In summary, we have established that two families of protecting groups for amines can also serve as effecting directing groups, thereby enabling enantioselective Suzuki reactions of unactivated secondary alkyl halides. Thus, a readily available nickel catalyst achieves asymmetric alkyl–alkyl cross-couplings of racemic carbamate- and sulfonamide-protected halo amines in good ee at room temperature. In contrast with our earlier method for the catalytic asymmetric synthesis of amines, which exclusively produced arylamines and was nitrogen-directed,2c for carbamate- and sulfonamide-directed cross-couplings, oxygen is the likely site of coordination to nickel. In particular, a structure-enantioselectivity study of sulfonamides is consistent with binding by oxygen, as is our observation that sulfones can function as a directing group in these stereoconvergent Suzuki reactions. This investigation has thus established for the first time that sulfonamides and sulfones can serve as effective directing groups in metal-catalyzed asymmetric carbon–carbon bond-forming processes. With respect to the mechanism of nickel-catalyzed Suzuki cross-couplings of unactivated secondary alkyl electrophiles, we have determined that the stereochemistry of the boron-bound carbon of a chiral trialkylborane is preserved in the reaction product, which is consistent with transmetalation with retention of stereochemistry and with structural integrity for the resulting nickel–carbon bond in subsequent stages of the catalytic cycle. A wide range of additional studies of cross-coupling reactions of alkyl electrophiles are underway.
Supplementary Material
ACKNOWLEDGMENT
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01–GM62871) and the University of Basilicata (fellowship to F. T.). We thank Dr. Jeffrey H. Simpson (Department of Chemistry Instrumentation Facility) for assistance with NMR spectroscopy.
Footnotes
ASSOCIATED CONTENT
Supporting Information Available: Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.For reviews and leading references on directed reactions, see: Hoveyda AH, Evans DA, Fu GC. Chem. Rev. 1993;93:1307–1370. Rousseau G, Breit B. Angew. Chem. Int. Ed. 2011;50:2450–2494. doi: 10.1002/anie.201006139.
- 2.(a) Aromatic ring: Saito B, Fu GC. J. Am. Chem. Soc. 2008;130:6694–6695. doi: 10.1021/ja8013677. (b) Oxygen of a carbonyl group: Owston NA, Fu GC. J. Am. Chem. Soc. 2010;132:11908–11909. doi: 10.1021/ja105924f. (c) Nitrogen of an arylamine: Lu Z, Wilsily A, Fu GC. J. Am. Chem. Soc. 2011;133:8154–8157. doi: 10.1021/ja203560q. (d) Amide: Zultanski SL, Fu GC. J. Am. Chem. Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515.
- 3.For reviews and leading references on cross-couplings of alkyl electrophiles, including enantioselective processes, see: Rudolph A, Lautens M. Angew. Chem. Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. Glorius F. Angew. Chem. Int. Ed. 2008;47:8347–8349. doi: 10.1002/anie.200803509. (c) Reference 2b.
- 4. Cordell GA, editor. The Alkaloids: Chemistry and Biology. San Diego: Elsevier; 2010. (b) For an overview of methods for the asymmetric synthesis of amines, see: Nugent TC, editor. Chiral Amine Synthesis. Weinheim, Germany: Wiley–VCH; 2010.
- 5.Koller M, Szinicz L. Chemical Warfare Agents. In: Clinical Toxicological Analysis. Külpmann W-R, editor. Vol. 2. Weinheim, Germany: Wiley–VCH; 2009. pp. 679–743. [Google Scholar]
- 6.For enantioselective Suzuki reactions of carbamate-protected halo alcohols (halohydrins), see Reference 2b.
- 7.For leading references to nickel-catalyzed Suzuki cross-couplings of: (a) Aryl carbamates and aryl methyl ethers: Yu D-G, Li B-J, Shi Z-J. Acc. Chem. Res. 2010;43:1486–1495. doi: 10.1021/ar100082d. (b) Aryl carbamates: Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J. Am. Chem. Soc. 2011;133:6352–6363. doi: 10.1021/ja200398c. (c) Aryl methyl ethers: Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447. (d) Oxygen leaving groups: Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t.
- 8.Notes: (a) Under the standard asymmetric cross-coupling conditions: (1) lower ee and/or yield are obtained when arylsulfonamides in which the aromatic group is hindered (mesityl) or very electron-poor (nosyl) are employed as substrates; (2) during the course of a reaction, the unreacted electrophile remains racemic, and the ee of the product is constant; (3) essentially no carbon–carbon bond formation is observed in the absence of NiBr2•diglyme, the ligand, or KOt-Bu. (b) For the stereoconvergent Suzuki reaction illustrated in entry 3 of Table 2: (1) the use of TBME, Et2O, or toluene, rather than i-Pr2O, as the solvent results in formation of the product in comparable ee, but somewhat diminished yield (~65%); (2) on a gram scale, the coupling proceeds in 90% ee and 79% yield; (3) with 5% NiBr2•diglyme/6% ligand, the cross-coupling product is generated in 90% ee and 42% yield; (4) the reaction is not highly water-sensitive (the addition of 0.1 equiv of water leads to no loss in ee or yield); (5) use of 1.3 equiv of alkylborane results in comparable enantioselectivity (89%), but lower yield (53%).
- 9.For examples of nickel-catalyzed Suzuki cross-couplings of aryl fluorides (perfluorinated arenes), see: Schaub T, Backes M, Radius U. J. Am. Chem. Soc. 2006;128:15964–15965. doi: 10.1021/ja064068b.
- 10.In a preliminary study, when the R group of the sulfone was Me or aryl, lower enantioselectivity was observed (~80% ee).
- 11.A homologue ((t-Bu)SO2(CH2)3CHClMe) cross-coupled in 49% ee and 85% yield.
- 12.For a previous report of the formation of an “ate” complex under somewhat different conditions, see: Saito B, Fu GC. J. Am. Chem. Soc. 2007;129:9602–9603. doi: 10.1021/ja074008l.
- 13.(a) For related mechanistic proposals for Ni/terpyridine-catalyzed Negishi reactions, see: Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J. Am. Chem. Soc. 2006;128:13175–13183. doi: 10.1021/ja063334i. Lin X, Phillips DL. J. Org. Chem. 2008;73:3680–3688. doi: 10.1021/jo702497p. (b) For an overview of mechanistic issues regarding nickel-catalyzed cross-couplings of unactivated alkyl halides, see: Hu X. Chem. Sci. 2011;2:1867–1886.
- 14.For the cross-coupling illustrated in entry 3 of Table 2, we have determined that the rate law is first order in the nickel catalyst and in the organoborane, but zeroth order in the electrophile.
- 15.In order to simplify the analysis of the coupling constants, we chose to employ per-deuterated cyclohexyl bromide as the unactivated secondary alkyl electrophile.
- 16.For a pioneering example of the use of this coupling-constant strategy in the context of elucidating the mechanism of organometallic reactions, see: Bock PL, Boschetto DJ, Rasmussen JR, Demers JP, Whitesides GM. J. Am. Chem. Soc. 1974;96:2814–2825.
- 17.After our mechanistic study was complete, Jarvo independently reported that transmetalation in a nickel-catalyzed primary–primary Suzuki cross-coupling, under conditions initially described by us (Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2007, 129, 9602–9603), proceeds with retention of stereochemistry: Taylor BLH, Jarvo ER. J. Org. Chem. 2011;76:7573–7576. doi: 10.1021/jo201263r.
- 18.For analogous studies of the mechanism of transmetalation in palladium-catalyzed Suzuki reactions of aryl electrophiles, see: Ridgway BH, Woerpel KA. J. Org. Chem. 1998;63:458–460. doi: 10.1021/jo970803d. Matos K, Soderquist JA. J. Org. Chem. 1998;63:461–470. doi: 10.1021/jo971681s.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.