Figure 1.
A 40 aa Peptide of LamB SS-β1 Competes for Full-Length Preprotein and Activates the SecY Complex
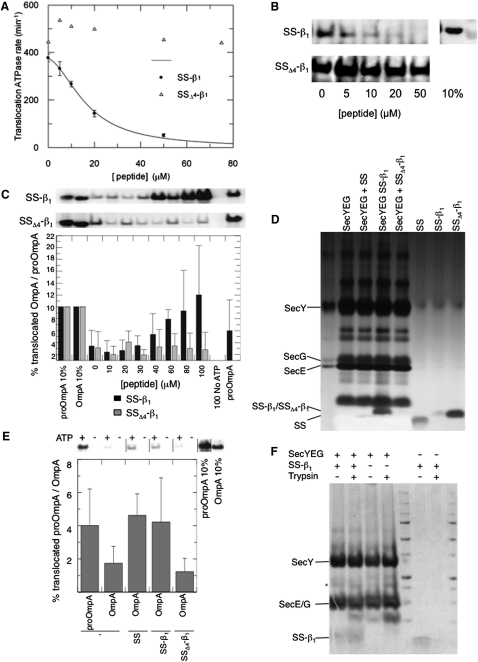
(A) Addition of LamB SS-β1, but not SSΔ4-β1, inhibits translocation-associated ATPase activity of SecA. ATPase rates were measured using the pyruvate kinase/lactate dehydrogenase linked assay in the presence of 50 nM SecA, 1 mM ATP, 1 μM SecYEG proteoliposomes, and 0.7 μM proOmpA after preincubation with increasing concentrations of the signal sequence peptides.
(B) Samples used in the ATPase assays shown in (A) were tested for translocation efficiency according to protease protection of proOmpA. Successfully translocated, protease-protected proOmpA was visualized by western blot. The top right-hand lane was loaded as a measure of 10% of the total proOmpA present in the samples.
(C) Translocation assays (as in B) using OmpA instead of proOmpA. The upper two panels show representative western blots for successfully translocated substrate in the presence of wild-type and mutant peptides; each lane corresponds to the bars in the quantification below, for increasing concentrations (0–100 μM) of peptides: SS-β1 (black bars) or SSΔ4-β1 (gray bars); n = 4–6. The translocation efficiency was calibrated against a 10% standard. Negative (no ATP with 100 μM peptide) and positive (proOmpA without peptide) controls are shown on the right. All error bars denote SD.
(D) SecYEG vesicles reconstituted in the presence of SS, SS-β1, or SSΔ4-β1 were loaded onto an SDS-PAGE gel and the polypeptides visualized by silver staining.
(E) Translocation assays for OmpA (as in B) using vesicles incorporating SecYEG with or without peptide. The upper panel shows a representative western, quantified (as in C) in the lower panel (n = 8). The peptide present in the initial reconstitution is indicated below. proOmpA was used as a positive control testing the competence of vesicles reconstituted without preprotein peptides (far left). All error bars denote SD.
(F) Coomassie-stained SDS-PAGE gel of 2D crystals of SecYEG grown in the presence or absence of SS-β1, before and after exposure to 1:100 (w/w) trypsin for 20 min at room temperature. ∗Well-known breakdown product of SecY (Collinson et al., 2001; Robson et al., 2007).