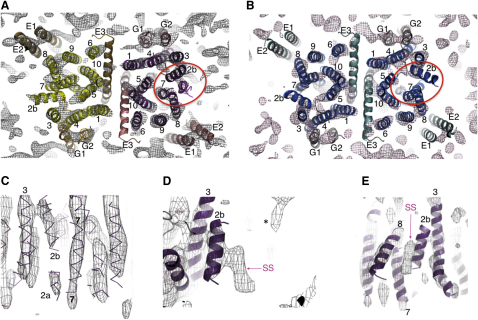
Figure 2.
Structure of SecYEG Bound to the Preprotein Peptide SS-β1
The TMS are labeled for SecY (1-10), SecE (E1-3), SecG (G1 and G2), and the signal sequence (SS). Maps are contoured at 1.5 SD.
(A and B) Top views from the cytoplasmic side of one crystalline membrane of SecYEG showing map density and super-imposed E. coli models (Experimental Procedures). The lateral gate of the substrate-occupied complex and its equivalent in the complex visualized without peptide are circled in red. (A) Structure of the SecYEG dimer bound to the preprotein mimic. The occupied complex with bound preprotein peptide is on the right-hand side of the dimer, with SecY, E, and G shown, respectively, in purple, salmon, and dark pink. The unoccupied complex is on the left-hand side of the dimer shown with SecY, E, and G in yellow, sand, and orange. (B) SecYEG, without bound preprotein peptide and without the applied 2-fold symmetry (Breyton et al., 2002), is shown for comparison with SecY, E, and G in blue, light teal, and gray.
(C–E) Detailed side views of map density of the SecYEG complex bound to the preprotein mimic (as in (A), right hand complex), with corresponding fitted E. coli homology model (purple lines). (C) view from the center of one SecYEG complex out through the lateral gate. (D) Side view toward TMS 2b and 3. ∗Denotes the density from the second crystal layer, which is not part of the structure being viewed. (E) looking into the lateral gate from the outside.