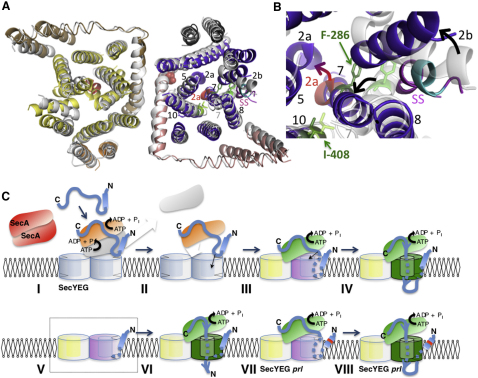
Figure 4.
Mechanism of Preprotein-Induced Activation of the SecY Complex
(A) Cytosolic view of the fitted models of SecYEG determined previously (Bostina et al., 2005) overlaid with the one bound by the preprotein peptide; color coding as in Figure 2, except that the SecYEG formerly determined without peptide is shown in white with the plug (2a) in red. The 4 residues -LAVA- of the signal sequence, which when deleted ablate preprotein transport and peptide activity (Figures 1A–1E), are shown in cyan. The sites associated with the signal sequence suppressor allele prlA4 (SecY-F286Y in TMS 7 and SecY-I408N in TMS 10) are shown in SecY determined without added peptide (lime green sticks on white ribbon) and in SecY bound to the preprotein mimic (dark green sticks on purple ribbon). SecE-S120 at the C-terminal periplasmic region of TMS 3, known to interact with the plug (2a; SecY-F67) (Flower et al., 1995; Tam et al., 2005), is represented by red spheres.
(B) Detail of the protein channel and lateral gate (as in A). The arrows describe the movement of TMS 2b, 7, and the plug (2a) associated with the binding of signal sequence (magenta, SS).
(C) Model for activation, channel opening and translocation. Preprotein (blue) with an N-terminal signal sequence (cylinder) is engaged by SecA (red inactive dimer) and targeted to the translocation machinery SecYEG (symmetrical light blue dimer). (I). The initiation of translocation involves ATP hydrolysis and the dissociation of SecA dimers (orange and white monomers) (Duong, 2003; Or et al., 2002; Robson et al., 2007), which exposes the signal sequence (II) to facilitate binding at the protein-lipid interface of SecYEG. (III). The association activates one monomer in the SecYEG dimer, breaking the 2-fold symmetry. The activated complex (as in A and B) is primed for translocation (purple), while the passive complex (yellow) becomes tightly closed and assists in the binding of SecA, now fully active (green) (Deville et al., 2011). (IV). ATP hydrolysis results in the intercalation of preprotein, channel opening (green) and translocation. (V). The activated asymmetric conformation can also be promoted by a trans-acting signal sequence peptide (dashed box) (Figures 1C–1E) and is visualized by the structure described here. (VI). In this bound state it is capable of transporting signal sequence-less substrates. (VII and VIII). The prlA mutants are predisposed to the activated form of SecYEG (purple) and capable of translocating proteins with defective signal sequences (red band on blue cylinder).