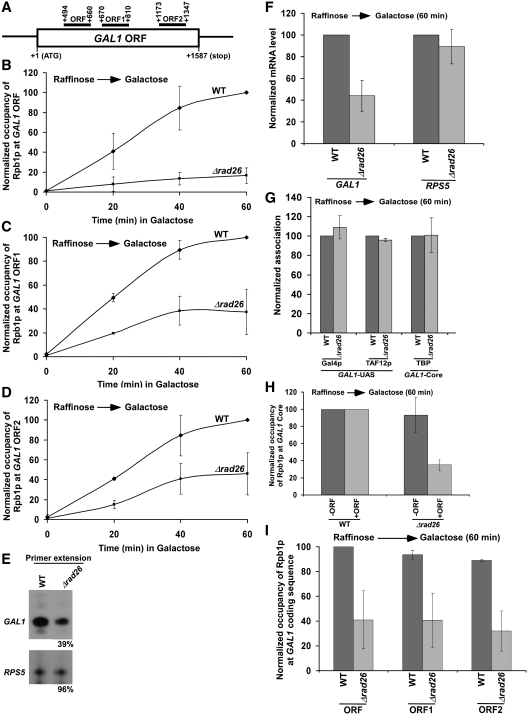
Figure 1.
Rad26p promotes the association of RNA polymerase II with the GAL1 coding sequence following transcriptional induction. (A) The schematic diagram of the GAL1 coding sequence or ORF with the PCR amplification regions (ORF, ORF1 and ORF2) in the ChIP assay. The numbers are presented with respect to the position of the first nucleotide of the initiation codon (+1). (B–D) Analysis of Rpb1p (the largest subunit of RNA polymerase II) association with the GAL1 coding sequence in the Δrad26 strain and its isogenic wild-type equivalent. The wild-type and Δrad26 strains were first grown in YPR up to an OD600 of 0.9, and then shifted to YPG for different periods of induction prior to formaldehyde-based in vivo cross-linking. Immunoprecipitation was performed using the mouse monoclonal antibody 8WG16 (Covance) against Rpb1p-CTD (carboxy terminal domain). The specific primer pair targeted to the different locations in the GAL1 coding sequence was used for PCR analysis of the immunoprecipitated DNA samples. The ChIP signal is the ratio of immunoprecipitate over the input in the autoradiogram. The maximum ChIP signal in the wild-type strain was set to 100, and other ChIP signals in the wild-type and Δrad26 strains were normalized with respect to 100. The normalized ChIP signals (represented as normalized occupancy) were plotted as a function of induction times. (E) Primer extension analysis. Both the wild-type and Δrad26 strains were grown in YPR up to an OD600 of 0.9, and then switched to YPG for 60 min. Total RNA was prepared and analyzed by primer extension. (F) RT-PCR analysis. Yeast strains were grown as in panel E. Total RNA was prepared and analyzed for transcription. Oligo(dT) primer was used for cDNA synthesis. (G) Rad26p does not regulate the formation of transcription complex at the GAL1 promoter. Both the wild-type and Δrad26 strains were grown as in panel E prior to formaldehyde-based in vivo cross-linking. Immunoprecipitations were performed using anti-Gal4p (RK5C1; Santa Cruz Biotechnology), anti-TAF12p (obtained from the laboratory of Michael R. Green) and anti-TBP (obtained from the laboratory of Michael R. Green) antibodies against Gal4p, TAF12p and TBP, respectively. (H) Regulation of Rpb1p occupancy at the GAL1 core promoter by Rad26p in the presence and absence of the coding sequence. Wild-type and mutant strains were grown as in panel E prior to cross-linking. +ORF, with open reading frame; and –ORF, without open reading frame. (I) Analysis of Rpb1p occupancy at different regions of the GAL1 coding sequence following 60 min transcriptional induction in YPG. The maximum ChIP signal was set to 100, and other ChIP signals were normalized with respect to 100.