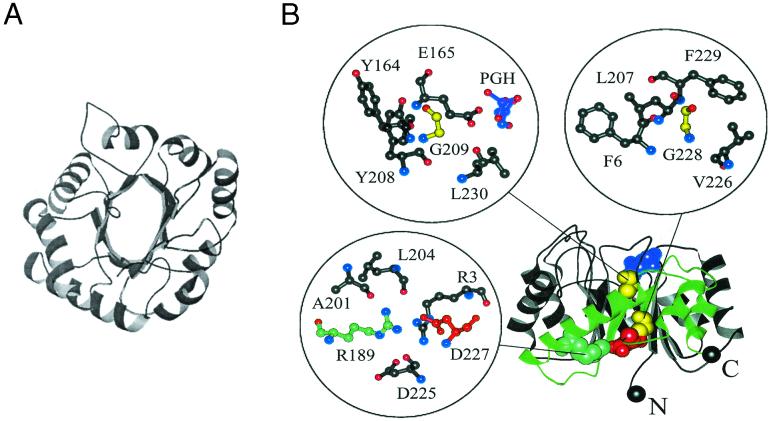
Figure 1.
Two views of the β/α barrel structure in TIM. (A) A view from the top of the structure showing the symmetry of the eight β/α domains. The N and C termini are depicted in black spheres. The active site in this orientation is facing the reader. (B) A side view showing the hydrophobic β-β-sheet interface and the four sites in the enzyme that are most sensitive to substitutions in the study by Silverman et al. (3). Insets show expanded views of the local structural environments around each residue. Phosphoglycolohydroxamate (PGH), a substrate analog included in the crystal structure, is shown in purple. Glycines 209 and 228 are colored yellow, R189 is colored cyan, and D227 is colored red. The region of the backbone proposed to be an independently folding subdomain is colored green.