Abstract
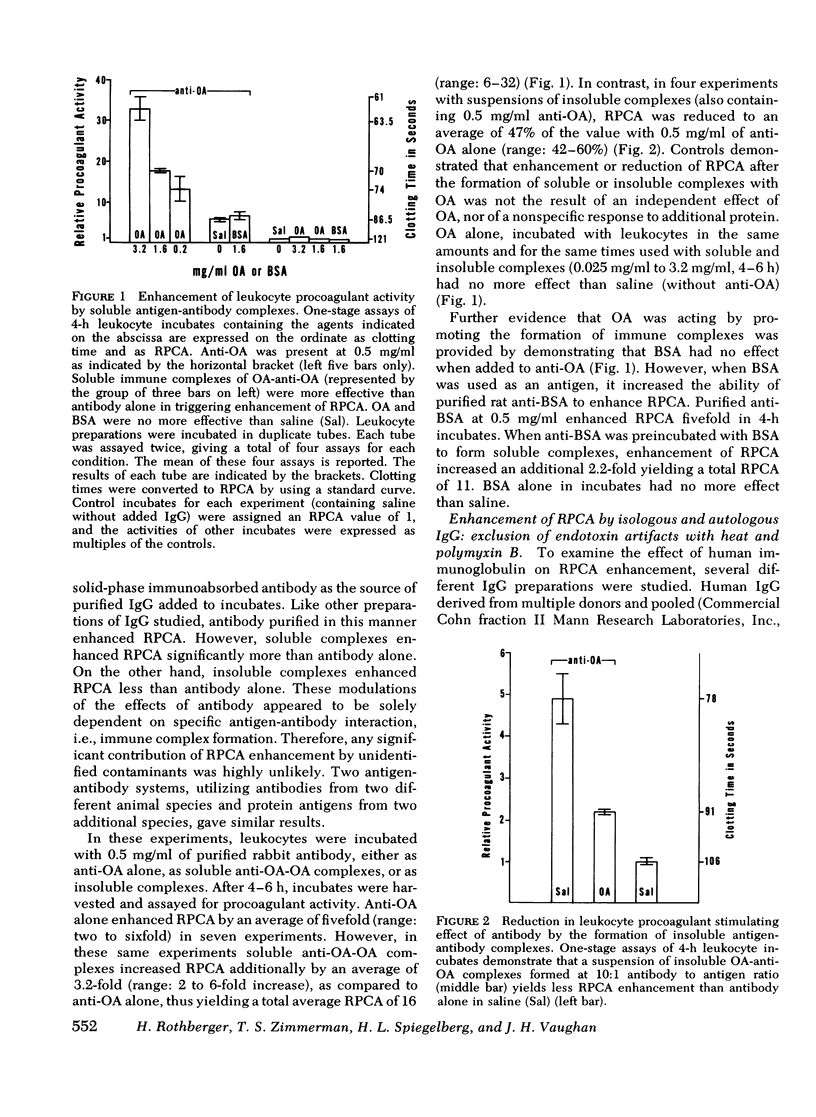
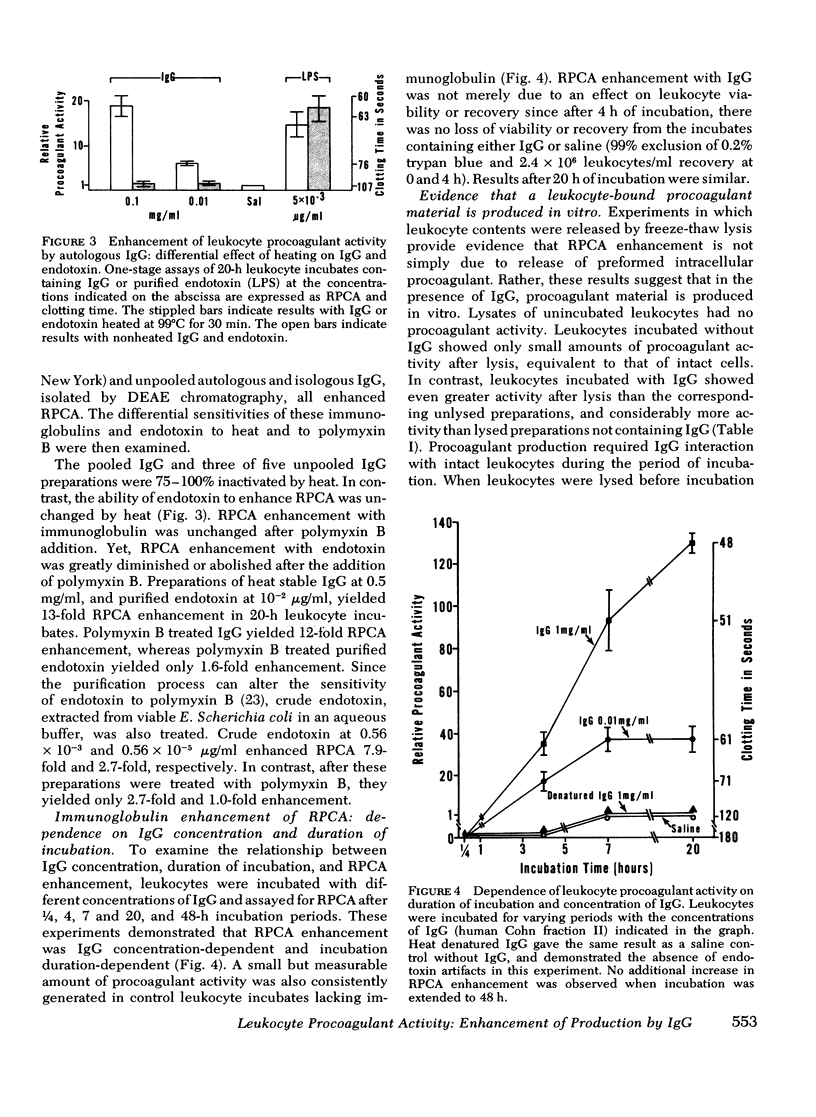
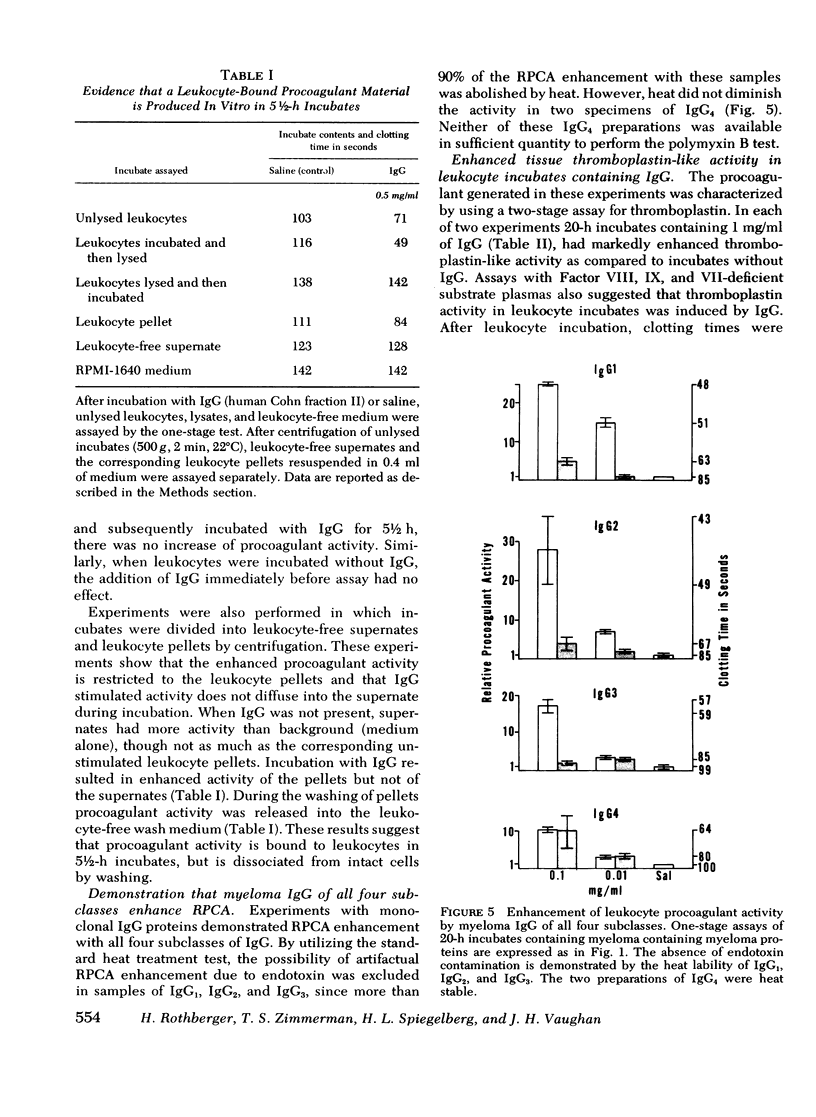
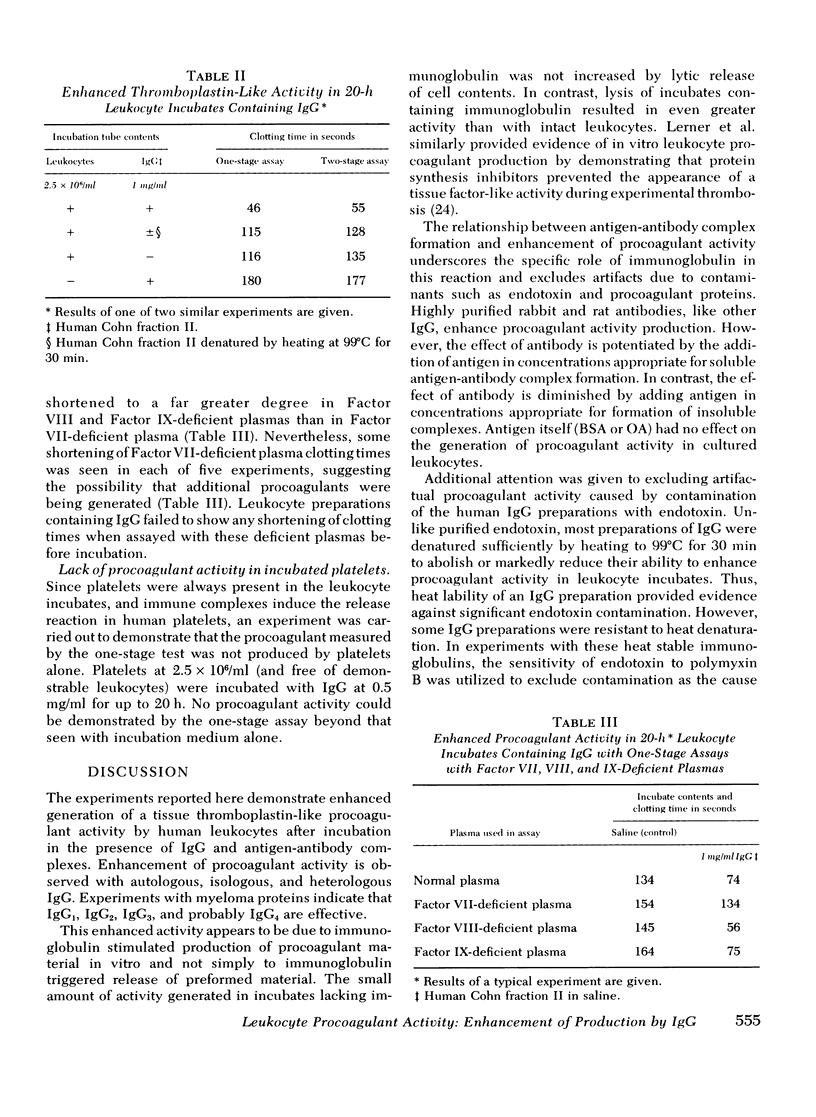
In a variety of immunologic diseases, fibrin-fibrinogen and immune complexes deposit in areas of tissue damage. However, the mechanisms which initiate fibrin-fibrinogen deposition have not been clarified. We find that the procoagulant activity of human leukocytes is markedly increased after incubation with immunoglobulin and immune complexes. This procoagulant activity is evident after 4-24 h incubation in the presence of as little as 0.1 mg/ml of autologous, isologous, or heterologous IgG. At least three of the four subclasses of IgG myeloma proteins are effective. Experiments with purified rabbit and rat antibodies demonstrate that enhancement of procoagulant activity is significantly greater with soluble antigen-antibody complexes than with immunoglobulin alone. In contrast, insoluble complexes are less affective than immunoglobulin alone. Artifacts due to endotoxin contamination of the IgG preparations were excluded on the basis of the differential sensitivities of immunoglobulin and endotoxin to heat and polymyxin B. Evidence is also presented which shows that enhancement of procoagulant activity involves the production, rather than a simple release, of leukocyte procoagulant activity in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Braun W. E., Merrill J. P. Urine fibrinogen fragments in human renal allografts. A possible mechanism of renal injury. N Engl J Med. 1968 Jun 20;278(25):1366–1371. doi: 10.1056/NEJM196806202782503. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chiller J. M., Weigle W. O. Cellular events during induction of immunologic unresponsiveness in adult mice. J Immunol. 1971 Jun;106(6):1647–1653. [PubMed] [Google Scholar]
- Colman R. W., Braun W. E., Busch G. J., Dammin G. J., Merrill J. P. Coagulation studies in the hyperacute and other forms of renal-allograft rejection. N Engl J Med. 1969 Sep 25;281(13):685–691. doi: 10.1056/NEJM196909252811301. [DOI] [PubMed] [Google Scholar]
- Cronlund M., Hardin J., Burton J., Lee L., Haber E., Bloch K. J. Fibrinopeptide A in plasma of normal subjects and patients with disseminated intravascular coagulation and systemic lupus erythematosus. J Clin Invest. 1976 Jul;58(1):142–151. doi: 10.1172/JCI108443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kanyerezi B. R., Lwanga S. K., Block K. J. Fibrinogen degradation products in serum and urine of patients with systemic lupus erythematosus. Relation to renal disease and pathogenetic mechanism. Arthritis Rheum. 1971 Mar-Apr;14(2):267–275. doi: 10.1002/art.1780140213. [DOI] [PubMed] [Google Scholar]
- Kincaid-Smith P. Coagulation and renal disease. Kidney Int. 1972 Oct;2(4):183–190. doi: 10.1038/ki.1972.93. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Lerner R. G., Goldstein R., Cummings G., Lange K. Stimulation of human leukocyte thromboplastic activity by endotoxin. Proc Soc Exp Biol Med. 1971 Oct;138(1):145–148. doi: 10.3181/00379727-138-35848. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Aptekar R. G., Steinberg A. D., Gralnick H. R., Decker J. L. Urinary fibrin split products in lupus nephritis. Correlation with other parameters of renal disease. Arthritis Rheum. 1974 Mar-Apr;17(2):158–164. doi: 10.1002/art.1780170208. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968 Jan;47(1):72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemetz J. Coagulant activity of leukocytes. Tissue factor activity. J Clin Invest. 1972 Feb;51(2):307–313. doi: 10.1172/JCI106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemetz J., Marcus A. J. The stimulatory effect of platelets and platelet membranes on the procoagulant activity of leukocytes. J Clin Invest. 1974 Dec;54(6):1437–1443. doi: 10.1172/JCI107891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemetz J., Nossel H. L. Activated coagulation factors: in-vivo and in-vitro studies. Br J Haematol. 1969 Apr;16(4):337–351. doi: 10.1111/j.1365-2141.1969.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Rickles F. R., Hardin J. A., Pitlick F. A., Hoyer L. W., Conrad M. E. Tissue factor activity in lymphocyte cultures from normal individuals and patients with hemophilia A. J Clin Invest. 1973 Jun;52(6):1427–1434. doi: 10.1172/JCI107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams W. M., Jr, Thorne E. G., Small P., Mass M. F., McIntosh R. M., Stanford R. E. Leukocytoclastic vasculitis. Arch Dermatol. 1976 Feb;112(2):219–226. [PubMed] [Google Scholar]
- Schroeter A. L., Copeman P. W., Jordon R. E., Sams W. M., Jr, Winkelmann R. K. Immunofluorescence of cutaneous vasculitis associated with systemic disease. Arch Dermatol. 1971 Sep;104(3):254–259. [PubMed] [Google Scholar]
- Vassalli P., McCluskey R. T. The pathogenetic role of the coagulation process in glomerular diseases of immunologic origin. Adv Nephrol Necker Hosp. 1971;1:47–63. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]


