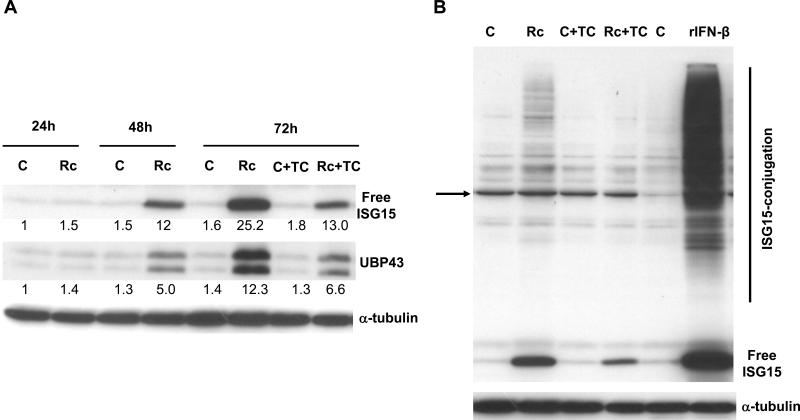
Figure 2.
R. conorii infection of HMECs up-regulates ISG15 and UBP43 protein expression and ISGylation. A. Western blot anaIysis of ISG15 and UBP43 in HMECs infected with R. conorii for different durations of time (24, 48, and 72 h). The antibody for UBP43 was capable of detecting both full length (39kDa) and amino-terminal truncated (34kDa) isoforms of UBP43. Also shown in the effect of tetracycline (TC) treatment on the levels of ISG15 and UBP43 expression during R. conorii infection. The results of a typical experiment (n=3) are presented. The relative intensity of each band in comparison to the level of baseline expression in uninfected controls is also shown. B. Evidence for protein ISGylation during R. conorii infection and IFN-β treatment of HMECs. Protein extracts were prepared from HMECs infected with R. conorii, treated with recombinant human IFN-β, or those left uninfected and untreated for controls. Equal amounts of protein were separated on an SDS-PAGE gel and electro-transferred to a nitrocellulose membrane. The membrane was then probed with an anti-ISG15 antibody. The arrow indicates a non-specific, cross-reactive protein band present in all experimental conditions, the intensity of which is variable even among simultaneously processed controls. The levels of free ISG15 in response IFN-β treatment and infection in the presence and absence of tetracycline and the effect of inhibition of rickettsial replication on ISGylation are also presented.